Medical News, Health News Latest, Medical News Today - Medical Dialogues |
AbbVie, Teva finalize USD 6.6 billion US opioid settlements
US: Teva Pharmaceutical Industries Ltd and AbbVie Inc have finalized the terms of settlements worth more than $6.6 billion to resolve thousands of lawsuits by U.S. state and local governments over the marketing of opioid painkillers, the companies and lawyers for the governments said Tuesday.
Under the deals, first announced in July, Israel-based Teva will pay up to $4.25 billion, including a supply of the overdose drug naloxone. AbbVie will pay up to $2.37 billion. The final amounts of the settlements will depend on how many state and local governments opt into them.
Lead attorneys for state and local governments in a joint statement called the deals "significant steps forward in our continued efforts to hold those responsible for the opioid epidemic accountable and obtain the necessary resources to battle its catastrophic effects."
Teva and AbbVie did not admit wrongdoing. The two companies said that as part of the settlements, they have resolved a dispute between them over responsibility for claims stemming from generic opioid business that Allergan sold to Teva in 2016.
The sprawling litigation over opioids, which began in 2017, has yielded more than $40 billion in settlements with drugmakers, distributors, and pharmacy chains.
Read also: AbbVie receives EMA Committee positive opinion for Crohn's Disease treatment Risankizumab
State and local authorities have said they will use the money to combat the opioid crisis, which according to federal government data has caused nearly 650,000 overdose deaths since 1999 and is continuing to worsen.
Overdoses involving opioids, including prescription pills and heroin, surged during the COVID-19 pandemic, increasing 38% in 2020 over the previous year and another 15% in 2021, according to the U.S. Centers for Disease Control and Prevention.
Read also: AbbVie to pay up to USD 2.37 billion to resolve US opioid claims
2 years 8 months ago
News,Industry,Pharma News,Latest Industry News
As RSV spreads, Oregon officials urge families to take precautions this Thanksgiving - KATU
- As RSV spreads, Oregon officials urge families to take precautions this Thanksgiving KATU
- Health officials urge families with babies and toddlers to reconsider Thanksgiving plans due to RSV KGW.com
- Multnomah County health officials urge many families to reconsider Thanksgiving as RSV infections surge Oregon Public Broadcasting
- St. Vincent’s pediatric units shift to crisis care standards, joining OHSU’s and Legacy’s children’s hospitals OregonLive
- View Full Coverage on Google News
2 years 8 months ago
STAT+: Pharmalittle: FDA approves first gene therapy for hemophilia B; pharma’s reputation slips in U.S.
Happy Thanksgiving Eve from STAT reporter Andrew Joseph, filling in for the day. A note that this newsletter is taking a break Thursday and Friday, something to be thankful for indeed. Now, how to leave you before the big day?
Sir Pharmalot himself suggested we make a mention of where turkey futures stand this morning, in case anyone is trying to score a last-minute turkey at bargain prices. Instead, we’re more focused on gaming out our oven strategy for the big meal, with a particular priority on the pecan pie we’re on tap for. We are, after all, a former winner of STAT’s pie contest, so we have a reputation to uphold. A few more pieces of wisdom as we head into the holiday: Warm up appropriately for both your turkey trots and turkey feasts — we don’t want any injuries out there. Salads absolutely have a place on the Thanksgiving table (you need something bright and acidic to cut through everything else that’s rich and fatty; cranberry sauce should not have to shoulder that responsibility alone). And finally, no matter how full you get, there’s always room for dessert — and yes, for an extra helping of gratitude. Enjoy the holiday.
The U.S. Food and Drug Administration on Tuesday approved the first gene therapy to treat people with hemophilia B, an inherited bleeding disorder, STAT writes. The one-time treatment, called Hemgenix, was developed by the Dutch biotech company UniQure and will be marketed by CSL Behring, an Australian pharmaceutical company. Hemgenix will cost $3.5 million, making it the most expensive drug approved to date. The approval of Hemgenix provides physicians with a new, potentially curative treatment option for patients with severe hemophilia B, a rare bleeding disorder caused by a genetic mutation that prevents the body from producing sufficient quantities of a clotting protein called Factor IX.
2 years 8 months ago
Pharma, Pharmalot, pharmalittle, STAT+
Nationwide survey on perceptions of Human Papillomavirus
According to recent PAHO data, Grenada, Guyana, and Suriname have the highest incidence rates of cervical cancer in the region, approximately 7 times higher than in Canada and the USA
View the full post Nationwide survey on perceptions of Human Papillomavirus on NOW Grenada.
2 years 8 months ago
Health, cervical cancer, curlan campbell, hpv, human papillomavirus, kamilah thomas purcell, paho, pan american health organisation
Medical News, Health News Latest, Medical News Today - Medical Dialogues |
Uttarakhand Combines two rounds of NEET PG Counselling: SC allows candidates "Free Surrender" of Seats, allows extending State Mop-up Deadline
New Delhi: Taking note of the fact that the State Government combined Round 1 and Round 2 of NEET-PG Counselling into one Main Round of Counselling, the Supreme Court has recently offered the Postgraduate medical aspirants in Uttarakhand the opportunity of "free surrender" of seats and thereafter participate in the State mop up round which is undergoing currently.
Further, the top court bench comprising the Chief Justice and Justice Hima Kohli has also permitted the State of Uttarakhand to extend the mop up round by three days.
"In this view of the matter and in order to obviate prejudice to the students, we order and direct that the petitioner and similarly placed students who were unable to exercise their options at the end of round 1 of the State counselling shall not be held to be ineligible merely because the State of Uttarakhand combined both rounds 1 and 2 of the counselling rounds. These students will be permitted to withdraw from the seats which have been allotted to them as a "free surrender" without suffering any penalty and would be permitted to participate in the State mop up round which is under way. The State of Uttarakhand shall duly publicize the above directions so that other similarly placed students are made aware of the option which has been granted in terms of the present order," observed the Apex Court bench.
"The State of Uttarakhand is permitted to extend the State mop up round by a further period of three days," it added.
The judgment came while the Court was considering the plea by a PG medical aspirant who after clearing the NEET-PG 2022-2023 examination, appeared in the counselling process.
As per the scheme for counselling which has been prescribed by the National Medical Commission, the counselling authorities at the Central and State level are required to conduct two separate rounds of counselling- i.e. rounds 1 and 2 before the mop up rounds for the All India Quota (AIQ) and the State mop up round take place.
However, the court noted that in case of Uttarakhand, the State Government authorities conducted only one consolidated round of counselling. In case of Uttarakhand, both the first and second rounds have been combined as the main round of counselling.
Issuing a notification in this regard on October 18, 2022, the State Government had notified, "...NEET PG 2022 Candidates participating in Uttarakhand State Centralized Counseling are informed that due to unavoidable reasons of Uttarakhand State Centralized Counseling, the first phase counseling was not conducted, due to which the counseling board decided to do main phase and mop phase. As per the instructions of Government of India /NMC / MCC /, a candidate admitted in the institute after the second stage counseling cannot leave his seat.
In view of the above, the first phase and second phase counseling mentioned in the information brochure issued by the counseling board should be considered as zero and the time table and information for the mop phase counseling will be issued separately."
The bench took note of the fact that because of the deviation by the State of Uttarakhand from the prescribed process, the rights of petitioner and other similarly placed students have been prejudically affected. If there would have been two rounds of counselling, such candidates would have been eligible to exercise the options available at the end of round 1 such as "free exit" and "upgradation". However, since both these rounds have been combined together, the students have been deprived of these options.
In this regard, the bench perused the clause 11.2.7 of the NEET-PG 2022 Information Bulletin issued by the State of Uttarakhand. The concerned clause stated, "If a candidate has withdrawn the admission or left the allotted seat after confirming the admission during the process of round-I counselling. He/she will be eligible for round-II counselling & subsequent rounds of counselling. Instruction for round-II and subsequent round will be uploaded separately."
Taking note of this, the Supreme Court bench also referred to the previous judgment of the Apex Court in the case of Nihila P P v Medical Counselling Committee (MCC) and Others, where the top court had directed that candidates who have joined the allotted seat in round 2 and further rounds of counselling will not be allowed to resign and will also be ineligible to take part in further rounds of any type of counselling. This order had been reiterated in following orders and the Supreme Court had clarified that students who have joined in round 2 of the state quota or round 2 of the All India Quota shall not be eligible to participate in the mop up round for All India Quota.
At this outset, the bench opined that the State's decision of combining two rounds of counselling has affected the PG medical aspirants in Uttarakhand and observed,
"It is plainly apparent that the failure of the State of Uttarakhand to hold two separate rounds of counselling has caused serious prejudice inasmuch as the options which would have been available at the end of round 1 would be foreclosed and would be unavailable."
Therefore, providing relief to the PG aspirants in Uttarakhand, the Court clarified that students like the petitioners who could not exercise their options at the end of round 1 of the State counselling shall not be held to be ineligible just because Uttarakhand government combined two rounds.
Further, the court has permitted these students to withdraw from the seats as a "free surrender" and participate in the State mop up round. Meanwhile, the State of Uttarakhand has been allowed to extend the State mop up round by a further period of three days.
To view the order, click on the link below:
https://medicaldialogues.in/pdf_upload/supreme-court-free-surrender-191738.pdf
Also Read: SC directs States, UTs to complete 2nd Round of NEET PG Counselling by November 16
2 years 8 months ago
State News,News,Health news,Delhi,Uttrakhand,Medico Legal News,Government Policies,Medical Education,Medical Courses News,Medical Admission News,Top Medical Education News
Medical News, Health News Latest, Medical News Today - Medical Dialogues |
Takeda Dengue Vaccine TAK-003 gets USFDA priority review
Japan: Takeda has announced that the U.S. Food and Drug Administration (USFDA) has accepted and granted priority review of the Biologics License Application (BLA) for TAK-003, the company's investigational dengue vaccine candidate.
Japan: Takeda has announced that the U.S. Food and Drug Administration (USFDA) has accepted and granted priority review of the Biologics License Application (BLA) for TAK-003, the company's investigational dengue vaccine candidate. In the U.S., TAK-003 is being evaluated for the prevention of dengue disease caused by any dengue virus serotype in individuals 4 years through 60 years of age.
Dengue is a mosquito-borne virus endemic in more than 125 countries, including the U.S. territories of Puerto Rico, the U.S. Virgin Islands and American Samoa. Incidence of dengue has increased globally over the past two decades and is a leading cause of fever among travelers returning from Latin America, the Caribbean and Southeast Asia.
"If approved, we believe TAK-003 has the potential to become an important dengue prevention option for healthcare providers, and we continue to be encouraged by our discussions with the FDA," said Gary Dubin, M.D., president of the Global Vaccine Business Unit at Takeda. "This year, of the 888 dengue infections in the U.S., 96% were a result of travel to dengue endemic areas. Of the 316 dengue infections in U.S. endemic territories, 97% were locally transmitted. At Takeda, we are guided by our commitment to serving these affected populations and are fully committed to working with the FDA to advance this filing."
Takeda's tetravalent dengue vaccine candidate (TAK-003) is based on a live-attenuated dengue serotype 2 virus, which provides the genetic "backbone" for all four vaccine viruses.
The TAK-003 BLA is supported by safety and efficacy data from the pivotal Phase 3 Tetravalent Immunization against Dengue Efficacy Study (TIDES) trial, where the dengue vaccine candidate met its primary endpoint by preventing 80.2% of symptomatic dengue cases at 12 months. In addition, TAK-003 met its secondary endpoint by preventing 90.4% of hospitalizations at 18 months, and in an exploratory analysis, it demonstrated protection against dengue fever through 4.5 years (54 months) after vaccination. The TIDES exploratory analyses showed that throughout the 4.5-year study follow-up, TAK-003 prevented 84% of hospitalized dengue cases and 61% of symptomatic dengue cases in the overall population, including both seropositive and seronegative individuals.
Currently, TAK-003 has not been approved by the FDA or any other health authority outside of Indonesia. In October 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) recommended the approval of Takeda's dengue vaccine candidate, TAK-003, for the prevention of dengue disease caused by any serotype in individuals four years of age and older in Europe and in dengue-endemic countries participating in the parallel EU-M4all procedure. The final step in the path to approval in Europe is a Marketing Authorization decision from the EMA, which is expected in the coming months. Regulatory reviews will also progress in dengue-endemic countries in Latin America and Asia.
Read also: Takeda gets positive CHMP opinion recommending nod of Dengue Vaccine in EU, Dengue-endemic countries
2 years 8 months ago
News,Industry,Pharma News,Latest Industry News
Medical News, Health News Latest, Medical News Today - Medical Dialogues |
Sandoz, Teva Pharma plans to ramp up biosimilars production
New Delhi: Generic drug makers Teva Pharmaceutical Industries and Sandoz say they are planning a significant ramp-up in production of biosimilars – copies of high-priced drugs used to treat illnesses such as rheumatoid arthritis and cancer – aiming to increase their share of an expanding market.
More than 55 brand-name blockbuster biologic drugs, each with peak annual sales above $1 billion, are due to come off patent by the end of the decade, according to industry estimates.
Executives from Teva, and Sandoz said they are targeting top-selling biologics such as Humira, AbbVie Inc's top-selling arthritis drug, which came off-patent in Europe and is due to come off-patent in the U.S. next year. But both companies face commercial and regulatory challenges, especially in the U.S., where biosimilars have not resulted in dramatically lower prices for consumers.
Biologics are complex molecules cultivated inside living cells, making it impossible to manufacture exact copies, as is the case with conventional pharmaceuticals made from chemical compounds.
Use of brand-name biologics typically account for an outsize proportion of drug spending in wealthier countries.
One of the biggest makers of generic drugs, Israeli-based Teva said it aims eventually to secure a 10% global market share of biosimilars. The company has been grappling with a heavy debt load since a 2016 acquisition and lawsuits arising from the U.S. opioid epidemic.
Teva currently has three approved biosimilars and 13 in development.
"We are going full blast now," Teva Chief Executive Kåre Schultz said in an interview with Reuters.
He said the company was targeting "80% of what's going off-patent in the next 10 years" including big sellers like the cancer drug Keytruda.
A division of Novartis, Sandoz is currently the second biggest player after Pfizer Inc in the biosimilar market by gross sales globally, per IQVIA data, cited by Sandoz. (Amgen is in third place).
Sandoz has launched eight biosimilar drugs.
"We now have over 15 products in development, and in the next five years we would like to double the value of our marketed portfolio," chief scientific officer Claire D'Abreu-Hayling told Reuters, adding that the biologics they intend to target are "really obvious opportunities".
BLOCKBUSTERS COMING OFF PATENT
Novartis plans to spin off its Sandoz generics business in 2023. The Swiss drugmaker said the unit failed to attract a serious buyer earlier this year as it considered options for the unit's future.
The more than 55 blockbuster biologics coming off patent protection in the United States and Europe over the next decade account for more than $270 billion in expected peak annual sales, according to a McKinsey analysis.
The analysis projected the value of the global biosimilar market could more than triple to an estimated $74 billion by 2030.
Next year could bring a test case in the U.S. market, with the anticipated launch of at least six biosimilars for Humira, which brings in about $15 billion to $20 billion in annual sales and is approved for autoimmune conditions including rheumatoid arthritis, psoriasis and Crohn's disease.
Sandoz and Teva are both working on biosimilars for Humira.
But the crowded field raises a tough question: Should the companies target the biggest selling biologics such as Humira, or aim for smaller brands that will likely attract fewer players, said Barclays pharmaceuticals analyst Emily Field.
Teva aims to ensure it is one of the first three biosimilars on the market for any given biologic, according to Sven Dethlefs, executive VP, North America commercial. He said the company intended to kick off multiple biosimilar development programs but would halt production if it could not make the top three.
While going after Humira, Sandoz is also targeting drugs like Biogen's multiple sclerosis medicine Tysabri, which is used in a much smaller patient population. The company believes no other biosimilar is being actively developed for Tysabri, said Chief Operating Officer Pierre Bourdage.
Creating the only biosimilar in a particular market for a particular drug could be a win, said Joshua Harris, senior VP focused on pharmaceutical patent litigation at Burford Capital, a provider of commercial legal finance. "That's going to be a rare situation," he said.
A typical biosimilar costs $100 million to $300 million to develop and between six to nine years to win approval, according to McKinsey. About half of efforts launched across the U.S., European, and Japanese markets fail at the earliest stages, the report found.
Generics, which can be priced as much as 80% to 90% less than branded pills, barely cost a few million to develop.
Biosimilars are viewed as "better than traditional generics, but nowhere near as good as branded pharma," Field said.
Commercial prospects will also depend on the regulatory environment. While more than 50 biosimilars have been introduced into the European market, the United States has taken longer to set up a regulatory pathway for biosimilars.
European regulators consider all approved biosimilars on par with the original biologic, which has helped boost uptake. Biosimilars have taken the majority of market share from brand-name biologics in Europe and resulted in savings between 75% to 90% off the reference product prices, according to a 2021 report by Duke University's Margolis Center for Health Policy.
In the United States, the Food and Drug Administration (FDA) has approved 39 biosimilars and 22 products have been launched as of October, according to an Amgen analysis.
The FDA typically expects additional trial data before designating a biosimilar as "interchangeable" with the original biologic, which would allow it to be automatically replaced with a biosimilar at the pharmacy counter.
In a note last month, SVB Securities analysts predicted most U.S. payers will likely stick with branded Humira next year, but seriously consider switching patients to interchangeable biosimilars by 2024.
Biosimilars launched in the U.S. have only taken about 20% of the volume share of the biologics they are based on, according to the Duke Report, with knockoffs delivering discounts of about 30% to 40%.
Patent-focused court battles have stymied some launches of biosimilars. Aggressive pricing strategies from branded drug companies also helped neutralize the limited discounts initially offered by biosimilar makers
Read also: Former Sandoz Chief Richard Francis joins Teva Pharma as President, CEO
2 years 8 months ago
News,Industry,Pharma News,Latest Industry News
Reduce salt in your diet
Sodium controls fluid balance in our bodies and maintains blood pressure. Eating too much sodium, or salt, may raise blood pressure and cause fluid retention. This could lead to swelling of the legs and feet or even other health issues such as...
Sodium controls fluid balance in our bodies and maintains blood pressure. Eating too much sodium, or salt, may raise blood pressure and cause fluid retention. This could lead to swelling of the legs and feet or even other health issues such as...
2 years 8 months ago
How to eat healthily during the holidays
It can be difficult to stay healthy during the holidays. Thanksgiving is typically celebrated by feasting, followed by seconds, maybe thirds, and topped off with dessert. Of course, then you have the post-feast nap, and then an additional leftover...
It can be difficult to stay healthy during the holidays. Thanksgiving is typically celebrated by feasting, followed by seconds, maybe thirds, and topped off with dessert. Of course, then you have the post-feast nap, and then an additional leftover...
2 years 8 months ago
Medical News, Health News Latest, Medical News Today - Medical Dialogues |
Febuxostat demonstrates efficacy and renal safety in patients with gout and CKD
Peiyu Zhang et al conducted a study to explore the efficacy and renal safety of febuxostat in gout and stage 2-4 chronic kidney disease (CKD) and factors that correlated with target serum urate (SU).The article has been published in Rheumatology therapy journal.
A single-center retrospective study including male patients with gout and CKD was conducted. SU, the rate of SU < 360 umol/L (RAT), and renal safety were analyzed in subjects who received febuxostat over 44 weeks.
Key findings of the study were:
• 102 patients (stage 2 CKD: n = 27; stage 3 CKD: n = 70; stage 4 CKD: n = 5) were enrolled.
• The SU level reduced significantly over 44 weeks (600.76 ± 95.42 versus 405.52 ± 111.93 umol/ L; P <0.05), and RAT increased to 39.20%.
• The overall estimated glomerular filtration rate (eGFR) level improved over 44 weeks (52.05 ± 11.68 versus 55.46 ± 14.49 mL/min/ 1.73 cm2, p <0.05).
• An obvious improvement of eGFR was observed in stage 3 CKD, in patients with <= 1 risk factor (hypertension, diabetic mellitus, hyperlipidemia, or usage of non-steroidal anti-inflammatory drugs), and in patients with terminal SU <360 umol/L (p <0.05).
• Logistic regression analysis indicated that baseline SU level and body weight were correlated with RAT.
• Patients with SU<600 umol/L and body weight <=70 kg reached higher RAT (56.7%).
The authors concluded that – "In patients with gout and CKD, urate lowering treatment with febuxostat significantly reduces SU levels and could improve renal function. Significant improvement in renal function was achieved in patients with stage 3 CKD, without hypertension, diabetic mellitus, hyperlipidemia, or usage of NSAIDs. A ''treat to target'' therapy with febuxostat could obviously improve renal function. Besides, patients with baseline SU <600 umol/L and body weight B 70 kg were more likely to achieve target SU.
Further reading:
Febuxostat Therapy for Patients with Gout and Stage 2–4 CKD: a Retrospective Study
Peiyu Zhang, Mo Chen et al
Rheumatology Therapy (2022) 9:1421–1434
2 years 8 months ago
Orthopaedics,Orthopaedics News,Top Medical News
VIDEO: Speaker outlines real-world advantages, risks of Port Delivery System
NEW YORK — David Eichenbaum, MD, discusses insights gained from real-world cases using the Port Delivery System with ranibizumab in this presentation from OSN New York Retina.Eichenbaum provides an overview of the steps involved in the implantation of the device, now known as Susvimo (Genentech), and outlines the benefits and possible adverse events associated with its use.“PDS is the first com
mercially available sustained-release treatment for common retinal disease,” he said. “Reintroduction after recall will be a critical time, and we have to work together with each
2 years 8 months ago
Medical News, Health News Latest, Medical News Today - Medical Dialogues |
KNRUHS Announces Seat Matrix For Post Basic Bsc Nursing, Bsc Nursing Admissions, details
Telangana: Kaloji Narayana Rao University of Health Sciences (KNRUHS)
announced the seat matrix for Post Basic BSc Nursing and BSc Nursing admission.
Per the notice, KNRUHS has allocated 3793 seats for Post Basic
BSc Nursing and BSc Nursing admission. Post Basic BSc Nursing has 228 seats, and
BSc Nursing has 3793 seats allotted.
Detailed seat matrix –
Telangana: Kaloji Narayana Rao University of Health Sciences (KNRUHS)
announced the seat matrix for Post Basic BSc Nursing and BSc Nursing admission.
Per the notice, KNRUHS has allocated 3793 seats for Post Basic
BSc Nursing and BSc Nursing admission. Post Basic BSc Nursing has 228 seats, and
BSc Nursing has 3793 seats allotted.
Detailed seat matrix –
Post Basic BSc Nursing Under Competent Authority Quota Male
Candidates – 17 seats
Name of the College
Seat
Apollo College Of Nursing, Jublee Hills, Hyderabad
5
Eshwari Bai College Of Nursing, Marredpally, Secunderabad
3
Svs College Of Nursing. Yenugonda, Mahabubnagar
5
Vijaya Health Care College Of Nursing, Keesara, Rr Dist.
4
Post Basic BSc Nursing Under Competent Authority Quota
Female Candidates – 211 seats
Name of the college
Seat
Apollo College Of Nursing, Jublee Hills,
Hyderabad
13
Care Nampally College Of
Nursing, Banjara Hills, Hyderabad
12
Deepthi College Of Nursing,
Shanthi Nagar, Nalgonda
18
Eshwari Bai College Of Nursing,
Marredpally, Secunderabad
15
Holy Mary College Of Nursing, Alkapur,
Hyderabad
18
Indo-American College Of
Nursing, Banjara Hills, Hyederabad
18
Maruti Paramedical College
, Bhadrachelam
18
Rohini College Of Nursing, Hanamkonda
18
Svs College Of Nursing. Yenugonda,
Mahabubnagar
13
Thirumala College Of
Nursing, Bradipur, Nizamabad
18
Vijaya Health Care College Of
Nursing, Keesara, Rr Dist.
14
Yashoda College Of
Nursing, Saroornagar, Hyd Erabad
18
Yashoda College Of
Nursing, Medchal, Malkajigiri
18
BSc Nursing Under Competent Authority Quota Male Candidates
– 178 seats
College Names
Seat
Apollo College of Nursing, C/o. Apollo Hospital Campus,
Jubilee Hills, Hyderabad- 500 033.
10
Balaji Institute of Nursing, Laknepally (V), Narsampet
(M), Warangal Dist - 506 331
10
Chelmeda Anand Rao Insitute of Medical Sciences, College
of Nursing, Bommakal, Karimnagar District.
6
Eashwaribai Memorial College of Nursing, West MarredPally,
Secunderabad
7
Global Kasturiba College of Nursing, Venkataramana Colony,
Near KPHM, 9th Phase, Sherilingampally (M), Rangareddy Dist.,
5
Kamineni Institute of Medical Sciences, College of
Nursing, Sreepuram, Narketpally - Nalgonda
15
Mallareddycollege of Nursing, Hyd
10
Medha Institute of Nursing & Diagnostic Sciences,
Paidipalli, Hanamkonda Mandal, Waranagal Dist.
18
Medicity College of Nursing, Ghanpur (Village), Medchal
(Mandal),
Ranga Reddy District.
15
Navodaya College of Nursing, Behind Petrol Pump,
Padmavathy Colony, Mahaboobnagar
15
Prathima Relief College of Nursing, Hanamkonda
20
Princess Durru Shehvar College of Nursing, Academic block,
Princess Durru Shehver, Children's & General Hospital, Purani Haveli,
Hyderabad
6
Shadan Institute of Medical Sciences, College of Nursing,
Peerancheru, Himayat Sagar Road, Hyderabad
12
Sita Ramaiah College of Nursing, Suraram Colony, Jeedimetla,
HYDERABAD
9
Sri Ramachandra College of Nursing, Sainagar,
Vinayaknagar, Opp. Mannurikapu kalyana Mandapam, Nizamabad.
9
SRK Memorial College of Nursing, FCA Building, Near
Railway Station, Mancherial, Adilabad Dist.
3
Vijaya Health Care Academic Society, College of Nursing,
Godhamakunta Village, Keesara Mandal, R.R. Dist
8
BSc Nursing Under Competent Authority Quota Female
Candidates – 3387
College Names
Seat
Govt. College of Nursing, Gandhi
Hospital, Secunderabad,
50
Govt College of Nursing, RIMS
Adilabad 504001,
50
Government College of Nursing Jagtial,
40
Government College of Nursing,
MGM Hospital, WARANGAL
80
Govt. College of Nursing, Raj
Bhavan Road, Hyderabad.
60
Govt college of Nursing Siricilla
100
Government College of Nursing,
Bansuwada
100
Government College of Nursing,
Gadwal
100
Government College of Nursing,
Siddipet
100
Angel College of Nursing,
V.Venkataya palem (Post), Wyra Road,Khammam-507 318.
30
Apex College of Nursing, Vanasthalipuram
Main Road, RR Dist, Hyderabad - 500 070.
30
Apollo College of Nursing, C/o.
Apollo Hospital Campus, Jubilee Hills, Hyderabad-500 033.
50
Aware College of Nursing, Santhivanam,
Nagarjunasagar Road, HYDERABAD - 500 035.
30
B.Sc.College of Nursing
Muslim Maternity Zanana General Hospital, Osmanapura, Chagderghat,
Hyderabad
18
Balaji Institute of Nursing,
Laknepally (V), Narsampet (M),
Warangal Dist - 506 331
14
Care College of Nursing, Begumpet,
Hyderabad
30
Care Nampally College of Nursing,
Banjara Hills, Hyderabad
30
Chandana College of Nursing,
Suryapet, Nalgonda District.
30
Chelmeda Anand Rao Insitute
of Medical Sciences,
College of Nursing, Bommakal,
Karimnagar District.
54
Deepthi College of Nursing,
Hayathnagar, RR District
24
Deepthi College of Nursing,
Shanti Nagar, Nalgonda Town,
30
Devs College of Nursing, Ankireddypalli,
Keesara Mandal, R.R.Dist. (NEW)
30
Durgabai Deshmukh College of
B.Sc., Nursing,
C/o. Durgabai Deshmukh Hospital
&
Research Centre,
30
Eashwaribai Memorial College
of Nursing, West MarredPally, Secunderabad
23
Global Kasturiba College of
Nursing, Venkataramana Colony, Near KPHM, 9th Phase, Sherilingampally (M),
Rangareddy Dist.,
25
Gowthami College of
Nursing, Narasimhapuri Colony, Opp: Astalashmi Kaman, Hyderabad
30
Green Leaf College of Nursing,
MDR Arcade, Cantonement, Bowenpally, Secunderabad
30
Holy Mary College of Nursing,
Alkapoor, Hyderabad.
30
Image Madhapur College of Nursing,
Malkajigiri, Hyderabad
30
Indian College of Nursing,
Rajanagaram, Wanaparthy
24
Indo-American College of Nursing,
Banjara Hills,
Hyderabad
57
Jaya College of Nursing,
Hanamkonda, Warangal
25
JAYA College of BSc., Nursing
Hanamkonda, Warangal
30
JMJ College of Nursing, St. Theresa's Hospital Campus,
Sanathnagar, Hyderabad
30
Kalanjali College of Nursing,
Saleem Nagar Colony, Malakpet, Hyderabad
24
Kamineni Institute of Medical
Sciences, College of Nursing, Sreepuram, Narketpally - Nalgonda
45
Kamineni College of Nursing,
Kamineni Hospitals Ltd. Campus, L.B.Nagar, Hyderabad
30
Kinnera College of Nursing,
Khammam
24
KIMS COLLEGE OF B.Sc.(NURSING),
Ministers Road, SECUNDERABAD
- 11.
48
Mahila Dakshitha Samithi
and BML College of Nursing, Chanda Nagar, R.R Dist.
30
Mallareddycollege of Nursing,
Hyd
14
Mamatha College of Nursing,
MGH Campus,
Girriprasad Nagar,
Khammam
60
Mamata College of Nursing, Bachupally,
Hyderabad
60
Maruthi College of Nursing,
Indira Market Road, BHADRACHALAM Khammam Dist.
24
Maruti Para Medical Academy
College of Nursing, Bhadrachalam,
KHAMMAM District.
24
Medha Institute of Nursing &
Diagnostic Sciences, Paidipalli,
Hanamkonda Mandal,
Waranagal Dist.
6
Medicity College of Nursing,
Ghanpur (Village), Medchal (Mandal),
Ranga Reddy District.
45
Medwin College of Nursing, Rajratna
Towers,
Chiragali lane, Nampally, Hyderabad
24
MNR College of Nursing, MNR
Medical College Campus,
Fasalwadi, SANGAREDDY
60
Mother Krishnabai College of
Nursing, Musheerabad,
HYDERABAD
30
Mother Theresa College of B.Sc.
Nursing, Moula Ali, ECIL, Hyderabad
30
Navodaya College of Nursing,
Behind Petrol Pump, Padmavathy Colony, Mahaboobnagar
15
Nightingale College of Nursing
Devarakonda, Nalgonda
30
Owaisi College of Nursing, C/o.
Owaisi Hospital & Research
Centre Campus, Near DMRL X Road,
Kanchan Bagh, Hyderabad
30
Pier Giorgio Frassati College
of Nursing, Loyolanagar, Suryapet, Nalgonda
30
Pioneer College of Nursing,
Prashanthi Nagar, Hyderabad
30
Prathima College of Nursing,
Pratima Instt. Of Medical Sciences Campus, Nagunur Road, KARIMNAGAR
60
Prathima Relief College of Nursing,
Hanamkonda
40
Prashanthi College of Nursing,
Hanamkonda
30
Princess Durru Shehvar College
of Nursing,
Academic block, Princess Durru
Shehver, Children's & General Hospital, Purani
Haveli, Hyderabad
18
Pulipati College of Nursing,
Ammapalem, Konijerla, Khammam
24
Pulipati Prasad College of
Nursing, Khanapuram Haveli, Khammam Dist
30
Dr.Patnam Mahender Reddy College
of Nursing, Chevella
60
Rohini College of Nursing, #2-5-742,
Subedari, Hanumakonda, Warangal Dist
30
Rainbow
College of Nursing, Ghanpur, Medhal, RR
Dist.Hyderabad
30
RVM Nursing
College, Mulugu, Siddipet Dist
60
Santosh
College of Nursing, Vidyanagar, KARIMNAGAR
30
Shadan
Institute of Medical Sciences, College of Nursing, Peerancheru, Himayat Sagar
Road, Hyderabad
18
Shanthi
College of Nursing, Himayathnager, Hyderabad
30
Shivananda
College of Nursing, Ramnagar,
Karimnagar Dist.
30
Sigma College
of Nursing, 35, SD.Road, Secunderabad - 500 003
25
Sita Ramaiah
College of Nursing, Suraram Colony,
Jeedimetla, HYDERABAD
21
Sneha College
of Nursing, Block No. 2, Naya Nagar, Kodad, Nalgonda Dist.
30
Sri
Bhavana College of Nursing, Kesarapally (V), Thipaarthy (M), NALGONDA.
30
Sri Ramachandra
College of Nursing, Sainagar, Vinayaknagar,
Opp. Mannurikapu kalyana Mandapam,
Nizamabad.
21
Sri Sai
College of Nursing, Shanthi Nagar,
Near Vishnavi
School Complex, Nalgonda
24
Sri
Satyalaxmi College of Nursing, Malakpet,
Satya
Hospital premises, Nalgonda Cross
Road,
Hyderabad
24
Sri
Surya College of Nursing, Chengicherla, Near Uppal Bus Depot,
RR Dist.
24
Sri
Venkata Sai College of Nursing, Yenugonda Village, Mahaboobnagar.
60
SRK
Memorial College of Nursing, FCA Building, Near Railway Station, Mancherial, Adilabad
Dist.
27
St. Ann's
College of Nursing, Fathimanagar,
Warangal
- 506 004.
30
St. John
College of Nursing, Yellapur, Hasanaparthy (M), Warangal Dist.
24
Swarnanjali
College of Nursing, Bank Colony, Khammam
24
Surabhi
College of Nursing, Siddipet
60
Tirumala
College of Nursing, Tirumala Nagar,
Bardipur
(V), Dichpally (M),
Nizamabad
30
Tulasi
College of Nursing, Electronic Complex, Kushaiguda,
ECIL X
Road, Hyderabad
30
TSRTC College
of Nursing Tarnaka, Hyderabad
30
Vijay Marie
College of Nursing, Umanagar Colony,
Begumpet,
HYDERABAD
24
Vijaya
Health Care Academic Society, College of Nursing, Godhamakunta Village, Keesara
Mandal,
R.R. Dist
22
Yashoda
College of Nursing, Road No. 16, Green Park Colony, Saroor Nagar, HYDERABAD
60
Yashoda
Lakshmi College of Nursing, Raj Bhavan Road, Somajiguda, Hyderabad
60
Yashodha
College of Nursing, Gowdavalli, Medchal, Malkajigiri
60
To view the notices, click on the links below -
https://medicaldialogues.in/pdf_upload/bsc-nursing-competent-male-191646.pdf
https://medicaldialogues.in/pdf_upload/pb-bsc-nursing-compentent-male-191647.pdf
https://medicaldialogues.in/pdf_upload/pb-bsc-nursing-competent-female-191648.pdf
https://medicaldialogues.in/pdf_upload/bsc-nursing-competent-female-191645.pdf
2 years 8 months ago
State News,News,Telangana,Medical Education,Nursing education News,Latest Medical Education News
Treating Long Covid Is Rife With Guesswork
Medical equipment is still strewn around the house of Rick Lucas, 62, nearly two years after he came home from the hospital. He picks up a spirometer, a device that measures lung capacity, and takes a deep breath — though not as deep as he’d like.
Still, Lucas has come a long way for someone who spent more than three months on a ventilator because of covid-19.
Medical equipment is still strewn around the house of Rick Lucas, 62, nearly two years after he came home from the hospital. He picks up a spirometer, a device that measures lung capacity, and takes a deep breath — though not as deep as he’d like.
Still, Lucas has come a long way for someone who spent more than three months on a ventilator because of covid-19.
“I’m almost normal now,” he said. “I was thrilled when I could walk to the mailbox. Now we’re walking all over town.”
Dozens of major medical centers have established specialized covid clinics around the country. A crowdsourced project counted more than 400. But there’s no standard protocol for treating long covid. And experts are casting a wide net for treatments, with few ready for formal clinical trials.
It’s not clear just how many people have suffered from symptoms of long covid. Estimates vary widely from study to study — often because the definition of long covid itself varies. But the more conservative estimates still count millions of people with this condition. For some, the lingering symptoms are worse than the initial bout of covid. Others, like Lucas, were on death’s door and experienced a roller-coaster recovery, much worse than expected, even after a long hospitalization.
Symptoms vary widely. Lucas had brain fog, fatigue, and depression. He’d start getting his energy back, then go try light yardwork and end up in the hospital with pneumonia.
It wasn’t clear which ailments stemmed from being on a ventilator so long and which signaled the mysterious condition called long covid.
“I was wanting to go to work four months after I got home,” Rick said over the laughter of his wife and primary caregiver, Cinde.
“I said, ‘You know what, just get up and go. You can’t drive. You can’t walk. But go in for an interview. Let’s see how that works,’” Cinde recalled.
Rick did start working earlier this year, taking short-term assignments in his old field as a nursing home administrator. But he’s still on partial disability.
Why has Rick mostly recovered while so many haven’t shaken the symptoms, even years later?
“There is absolutely nothing anywhere that’s clear about long covid,” said Dr. Steven Deeks, an infectious disease specialist at the University of California-San Francisco. “We have a guess at how frequently it happens. But right now, everyone’s in a data-free zone.”
Researchers like Deeks are trying to establish the condition’s underlying causes. Some of the theories include inflammation, autoimmunity, so-called microclots, and bits of the virus left in the body. Deeks said institutions need more money to create regional centers of excellence to bring together physicians from various specialties to treat patients and research therapies.
Patients say they are desperate and willing to try anything to feel normal again. And often they post personal anecdotes online.
“I’m following this stuff on social media, looking for a home run,” Deeks said.
The National Institutes of Health promises big advances soon through the RECOVER Initiative, involving thousands of patients and hundreds of researchers.
“Given the widespread and diverse impact the virus has on the human body, it is unlikely that there will be one cure, one treatment,” Dr. Gary Gibbons, director of the National Heart, Lung, and Blood Institute, told NPR. “It is important that we help find solutions for everyone. This is why there will be multiple clinical trials over the coming months.”
Meanwhile, tension is building in the medical community over what appears to be a grab-bag approach in treating long covid ahead of big clinical trials. Some clinicians hesitate to try therapies before they’re supported by research.
Dr. Kristin Englund, who oversees more than 2,000 long covid patients at the Cleveland Clinic, said a bunch of one-patient experiments could muddy the waters for research. She said she encouraged her team to stick with “evidence-based medicine.”
“I’d rather not be just kind of one-off trying things with people, because we really do need to get more data and evidence-based data,” she said. “We need to try to put things in some sort of a protocol moving forward.”
It’s not that she lacks urgency. Englund experienced her own long covid symptoms. She felt terrible for months after getting sick in 2020, “literally taking naps on the floor of my office in the afternoon,” she said.
More than anything, she said, these long covid clinics need to validate patients’ experiences with their illness and give them hope. She tries to stick with proven therapies.
For example, some patients with long covid develop POTS — a syndrome that causes them to get dizzy and their heart to race when they stand up. Englund knows how to treat those symptoms. With other patients, it’s not as straightforward. Her long covid clinic focuses on diet, sleep, meditation, and slowly increasing activity.
But other doctors are willing to throw all sorts of treatments at the wall to see what might stick.
At the Lucas house in Tennessee, the kitchen counter can barely contain the pill bottles of supplements and prescriptions. One is a drug for memory. “We discovered his memory was worse [after taking it],” Cinde said.
Other treatments, however, seemed to have helped. Cinde asked their doctor about her husband possibly taking testosterone to boost his energy, and, after doing research, the doctor agreed to give it a shot.
“People like myself are getting a little bit out over my skis, looking for things that I can try,” said Dr. Stephen Heyman, a pulmonologist who treats Rick Lucas at the long covid clinic at Ascension Saint Thomas in Nashville.
He’s trying medications seen as promising in treating addiction and combinations of drugs used for cholesterol and blood clots. And he has considered becoming a bit of a guinea pig himself.
Heyman has been up and down with his own long covid. At one point, he thought he was past the memory lapses and breathing trouble, then he caught the virus a second time and feels more fatigued than ever.
“I don’t think I can wait for somebody to tell me what I need to do,” he said. “I’m going to have to use my expertise to try and find out why I don’t feel well.”
This story is from a reporting partnership that includes WPLN, NPR, and KHN.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
USE OUR CONTENT
This story can be republished for free (details).
2 years 8 months ago
COVID-19, Public Health, States, Audio, Chronic Disease Care, Clinics, Long Covid, Tennessee
News Archives - Healthy Caribbean Coalition
Sagicor Know Your Numbers Health and Wellness Campaign
Sagicor in collaboration with the Healthy Caribbean Coalition has produced the Know Your Numbers Health and Wellness Campaign video series which can be viewed below. Stay tuned every Thursday to learn more!
Introduction
Sagicor in collaboration with the Healthy Caribbean Coalition has produced the Know Your Numbers Health and Wellness Campaign video series which can be viewed below. Stay tuned every Thursday to learn more!
Introduction
Welcome to our Know Your Numbers series! Our resident nurse Rosanna Springer introduces the series by highlighting why it is important for us to Know Our Numbers.
Episode 1
Your doctor takes your blood pressure when you visit, or you sometimes take it yourself at home. But do you know what the numbers really mean? Dr. Khatija Mangera explains what the numbers mean and what they should be to maintain a healthy lifestyle.
Episode 2
When and how should you take your blood pressure correctly? Dr. Khatija Mangera demonstrates with Nurse Rosanna Springer and shares some steps you should take and some items you shouldn’t consume before taking your blood pressure.
Episode 3
In this video, Dr. Joseph Herbert highlights the impact your cholesterol has on all your risks factors, namely heart disease and stroke. Watch as he explains why cholesterol should be taken seriously as it can lead to a number of other medical conditions.
Episode 4
Have you ever heard about good cholesterol or only bad cholesterol? What would contribute to good cholesterol? Dr. Joseph Herbert provides a comprehensive explanation of both and explains how they work.
Episode 5
Blood sugar and HbA1C. Dr. Diane Brathwaite explains factors that affect your blood sugar and provides advice on the importance of knowing your levels throughout the day. Listen to hear about some activities that may impact these readings.
Episode 6
Taking blood sugar readings is something unique to each individual. Dr. Diane Brathwaite explains how the frequency relates to your personal medical condition. Watch as she shares some expert advice on taking blood sugar readings.
Episode 7
Do you know what your Body Mass Index (BMI) is or how it’s calculated? Join Dr. Kia Lewis in this video as she explains these two areas and also learn what the healthy and unhealthy ranges are with regards to BMI.
Episode 8
As we close off this Know Your Numbers video series, Dr. Kia Lewis provides advice on the ideal waist circumference for both men and women. An elevated waist circumference can put you at risk of disease. We encourage you to Know Your Numbers.
-->
More videos coming every Thursday so please come back next week!
The post Sagicor Know Your Numbers Health and Wellness Campaign appeared first on Healthy Caribbean Coalition.
2 years 8 months ago
Latest, News, Sagicor
Jamaica observes World Antimicrobial Awareness Week
JAMAICA has joined the rest of the world in the annual observance of World Antimicrobial Awareness Week (WAAW) from November 18 to 24, under the theme 'Preventing Antimicrobial Resistance Together'.
Jamaica's activities are being led by the Pan American Health Organization (PAHO), in conjunction with the Ministry of Industry, Investment and Commerce, Ministry of Agriculture and Fisheries, and the Ministry of Health and Wellness.
WAAW is a World Health Organization (WHO)-led global campaign that is celebrated annually to improve awareness and understanding of antimicrobial resistance and to encourage best practices among the public, as health stakeholders and policymakers all play a critical role in reducing the further emergence and spread of antimicrobial resistance (AMR).
AMR occurs when bacteria, viruses, fungi and parasites change over time and no longer respond to medicines, making infections harder to treat and increasing the risk of disease spread, severe illness and death. As a result of drug resistance, antibiotics and other antimicrobial medicines become ineffective and infections become increasingly difficult or impossible to treat.
According to the WHO, researchers estimated that AMR in bacteria caused an estimated 1.27 million deaths in 2019.
In an interview with JIS News, National PAHO Infection Prevention and Control (IPC)/AMR Consultant Dr Glendee Reynolds-Campbell explained that the main activity for WAAW will be a symposium which will be held under the WAAW theme. The event will take place on Thursday, November 24 beginning at 10:00 at the Regional Headquarters Building, The University of the West Indies.
The other activity for the week will be a webinar hosted by the Ministry of Agriculture and Fisheries, in collaboration with the Ministry of Industry, Investment and Commerce, on Wednesday, November 23.
2 years 8 months ago
PAHO/WHO | Pan American Health Organization
Canada contributes over U$ 11 million to PAHO’s initiative to strengthen the regional manufacturing of vaccines
Canada contributes over U$ 11 million to PAHO’s initiative to strengthen the regional manufacturing of vaccines
Cristina Mitchell
21 Nov 2022
Canada contributes over U$ 11 million to PAHO’s initiative to strengthen the regional manufacturing of vaccines
Cristina Mitchell
21 Nov 2022
2 years 8 months ago
Medical News, Health News Latest, Medical News Today - Medical Dialogues |
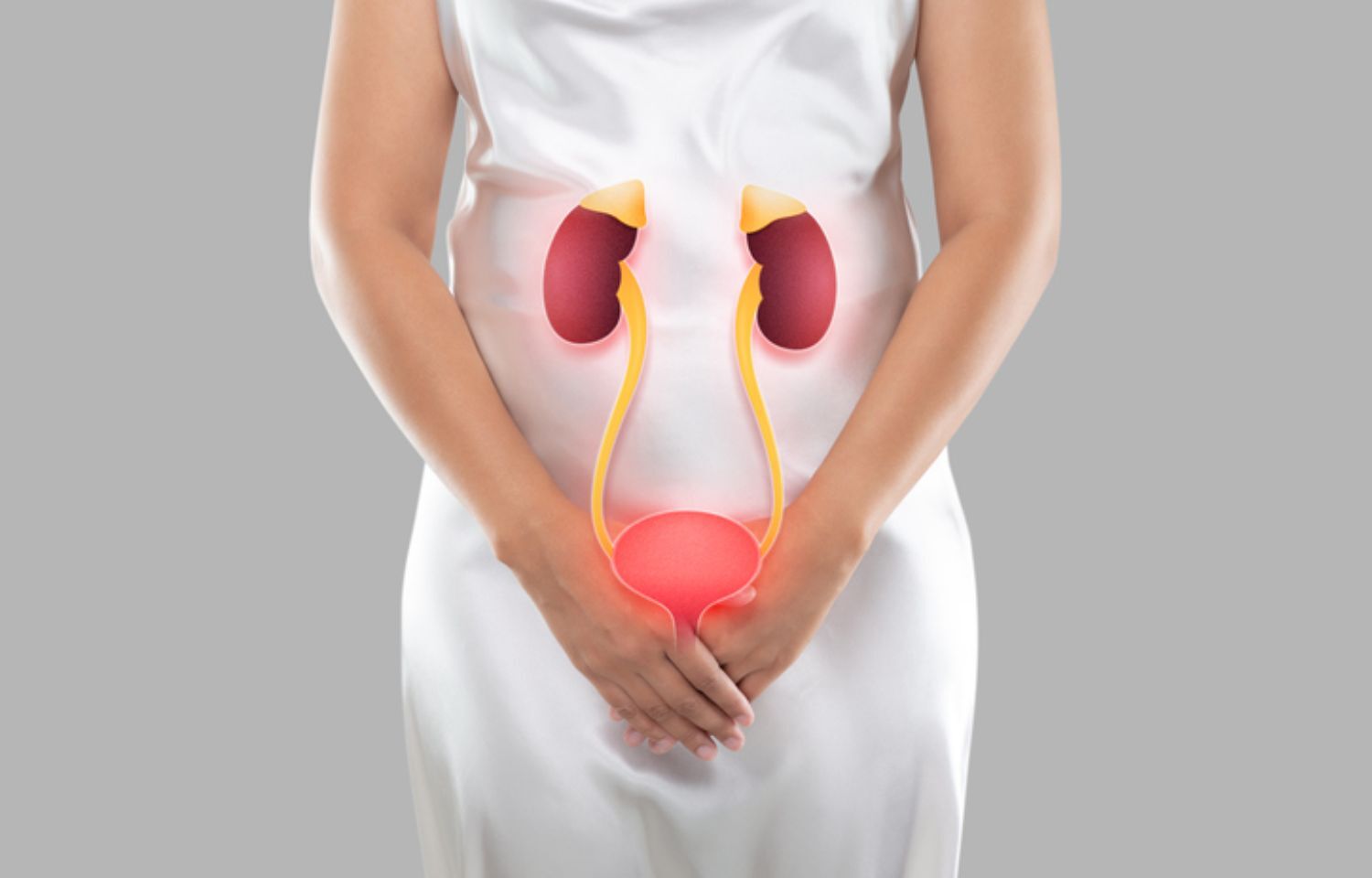
Regular exercise and weight-loss alleviate symptoms of lower urinary tract among diabetes patients
Palestine: Lower urinary tract symptoms (LUTS) are a noticeable cause of morbidity for people with diabetes mellitus, and regular exercise and weight loss techniques can aid to alleviate LUTS, states a study published in BMC UROLOGY.
Diabetes mellitus (DM) is a prevalent health issue in developing world. Lower urinary tract symptoms (LUTS), a hidden and enigmatic morbidity, are widespread in diabetic individuals. Patients with diabetes mellitus (DM) experience LUTS that has a complex pathogenesis. Importantly, LUTS is understood to lead to both mental and physical suffering.
Studies examining risk factors that might lead to LUTS or the prevalence of LUTS among DM patients have not been conducted in Palestine.
Therefore, given the frequency of LUTS and risk factors, the authors set out to investigate the LUTS problem among DM patients visiting primary health care facilities and to determine whether there may be a connection between LUTS and the quality of life of diabetics.
For this purpose, data from 378 diabetic individuals were gathered over a 6-month period, from May 2021 to October 2021, in primary healthcare facilities. Data were gathered using the Incontinence Impact Questionnaire-7 (IIQ-7), Urogenital Distress Inventory-6 (UDI-6), and demographic and clinical parameters. Included were all patients whose laboratory tests revealed they had DM. Patients having a confirmed diagnosis of a urogenital disorder, a background of urological surgery or recurring UTIs, or those suffering from a mental illness were disqualified. (29.9%) were in the age range of (58–67). Females made up 49%. A third of the cohort was obese, and 50% of the group was overweight. 81% had type 2 diabetes. Nearly all of them were receiving medical care. Both single-variate and multiple-variate analyses were conducted.
Key results of the study:
- The UDI-6 scale had a median score of 5.50 (2.00-8.00) and the IIQ-7 scale had a median score of 5 (0.00-10.00).
- Residency (p = 0.038) and regular exercise (p = 0.001) were substantially and negatively associated with the UDI-6 score, according to multiple linear regression models, whereas female gender (p = 0.042), insulin use (p = 0.009), and the presence of comorbidities (p = 0.007) were strongly and positively linked with this score.
- Age and body mass index (BMI) were considerably and favorably correlated with the IIQ-7 score (p = 0.040 and p< 0.001, respectively).
"LUTS is linked to high BMI and inactivity, regardless of the presence or absence of DM. It is also obvious that losing weight and exercising regularly help treat urine incontinence and alleviate LUTS in females. As a result, weight loss and more physical activity not only aid the patient's DM control and prevent serious complications, but also aid the patient's LUTS," asserted Faris Abushamma, Department of Medicine and team.
The findings support the notion that LUTS directly impairs diabetic patients' quality of life. The need for interventions to be implemented at all healthcare levels to identify such issues at an early stage in order to prevent physical and emotional tiredness among DM patients is compellingly demonstrated by this novel concept in Palestine.
REFERENCE
Qasrawi, H., Tabouni, M., Almansour, S.W. et al. An evaluation of lower urinary tract symptoms in diabetic patients: a cross-sectional study. BMC Urol 22, 178 (2022). https://doi.org/10.1186/s12894-022-01133-1
2 years 8 months ago
Diabetes and Endocrinology,Urology,Urology News,Top Medical News
Audits — Hidden Until Now — Reveal Millions in Medicare Advantage Overcharges
Newly released federal audits reveal widespread overcharges and other errors in payments to Medicare Advantage health plans for seniors, with some plans overbilling the government more than $1,000 per patient a year on average.
Summaries of the 90 audits, which examined billings from 2011 through 2013 and are the most recent reviews completed, were obtained exclusively by KHN through a three-year Freedom of Information Act lawsuit, which was settled in late September.
The government’s audits uncovered about $12 million in net overpayments for the care of 18,090 patients sampled, though the actual losses to taxpayers are likely much higher. Medicare Advantage, a fast-growing alternative to original Medicare, is run primarily by major insurance companies.
Officials at the Centers for Medicare & Medicaid Services have said they intend to extrapolate the payment error rates from those samples across the total membership of each plan — and recoup an estimated $650 million as a result.
But after nearly a decade, that has yet to happen. CMS was set to unveil a final extrapolation rule Nov. 1 but put that decision off until February.
Ted Doolittle, a former deputy director of CMS’ Center for Program Integrity, which oversees Medicare’s efforts to fight fraud and billing abuse, said the agency has failed to hold Medicare Advantage plans accountable. “I think CMS fell down on the job on this,” said Doolittle, now the health care advocate for the state of Connecticut.
Doolittle said CMS appears to be “carrying water” for the insurance industry, which is “making money hand over fist” off Medicare Advantage. “From the outside, it seems pretty smelly,” he said.
In an email response to written questions posed by KHN, Dara Corrigan, a CMS deputy administrator, said the agency hasn’t told health plans how much they owe because the calculations “have not been finalized.”
Corrigan declined to say when the agency would finish its work. “We have a fiduciary and statutory duty to address improper payments in all of our programs,” she said.
The 90 audits are the only ones CMS has completed over the past decade, a time when Medicare Advantage has grown explosively. Enrollment in the plans more than doubled during that period, passing 28 million in 2022, at a cost to the government of $427 billion.
Seventy-one of the 90 audits uncovered net overpayments, which topped $1,000 per patient on average in 23 audits, according to the government’s records. Humana, one of the largest Medicare Advantage sponsors, had overpayments exceeding that $1,000 average in 10 of 11 audits, according to the records.
CMS paid the remaining plans too little on average, anywhere from $8 to $773 per patient.
Auditors flag overpayments when a patient’s records fail to document that the person had the medical condition the government paid the health plan to treat, or if medical reviewers judge the illness is less severe than claimed.
That happened on average for just over 20% of medical conditions examined over the three-year period; rates of unconfirmed diseases were higher in some plans.
As Medicare Advantage’s popularity among seniors has grown, CMS has fought to keep its audit procedures, and the mounting losses to the government, largely under wraps.
That approach has frustrated both the industry, which has blasted the audit process as “fatally flawed” and hopes to torpedo it, and Medicare advocates, who worry some insurers are getting away with ripping off the government.
“At the end of the day, it’s taxpayer dollars that were spent,” said David Lipschutz, a senior policy attorney with the Center for Medicare Advocacy. “The public deserves more information about that.”
At least three parties, including KHN, have sued CMS under the Freedom of Information Act to shake loose details about the overpayment audits, which CMS calls Risk Adjustment Data Validation, or RADV.
In one case, CMS charged a law firm an advance search fee of $120,000 and then provided next to nothing in return, according to court filings. The law firm filed suit last year, and the case is pending in federal court in Washington, D.C.
KHN sued CMS in September 2019 after the agency failed to respond to a FOIA request for the audits. Under the settlement, CMS agreed to hand over the audit summaries and other documents and pay $63,000 in legal fees to Davis Wright Tremaine, the law firm that represented KHN. CMS did not admit to wrongfully withholding the records.
High Coders
Most of the audited plans fell into what CMS calls a “high coding intensity group.” That means they were among the most aggressive in seeking extra payments for patients they claimed were sicker than average. The government pays the health plans using a formula called a “risk score” that is supposed to render higher rates for sicker patients and lower ones for healthier ones.
But often medical records supplied by the health plans failed to support those claims. Unsupported conditions ranged from diabetes to congestive heart failure.
Overall, average overpayments to health plans ranged from a low of $10 to a high of $5,888 per patient collected by Touchstone Health HMO, a New York health plan whose contract was terminated “by mutual consent” in 2015, according to CMS records.
Most of the audited health plans had 10,000 members or more, which sharply boosts the overpayment amount when the rates are extrapolated.
In all, the plans received $22.5 million in overpayments, though these were offset by underpayments of $10.5 million.
Auditors scrutinize 30 contracts a year, a small sample of about 1,000 Medicare Advantage contracts nationwide.
UnitedHealthcare and Humana, the two biggest Medicare Advantage insurers, accounted for 26 of the 90 contract audits over the three years.
Eight audits of UnitedHealthcare plans found overpayments, while seven others found the government had underpaid.
UnitedHealthcare spokesperson Heather Soule said the company welcomes “the program oversight that RADV audits provide.” But she said the audit process needs to compare Medicare Advantage to original Medicare to provide a “complete picture” of overpayments. “Three years ago we made a recommendation to CMS suggesting that they conduct RADV audits on every plan, every year,” Soule said.
Humana’s 11 audits with overpayments included plans in Florida and Puerto Rico that CMS had audited twice in three years.
The Florida Humana plan also was the target of an unrelated audit in April 2021 by the Health and Human Services inspector general. That audit, which covered billings in 2015, concluded Humana improperly collected nearly $200 million that year by overstating how sick some patients were. Officials have yet to recoup any of that money, either.
In an email, Humana spokesperson Jahna Lindsay-Jones called the CMS audit findings “preliminary” and noted they were based on a sampling of years-old claims.
“While we continue to have substantive concerns with how CMS audits are conducted, Humana remains committed to working closely with regulators to improve the Medicare Advantage program in ways that increase seniors’ access to high-quality, lower cost care,” she wrote.
Billing Showdown
Results of the 90 audits, though years old, mirror more recent findings of a slew of other government reports and whistleblower lawsuits alleging that Medicare Advantage plans routinely have inflated patient risk scores to overcharge the government by billions of dollars.
Brian Murphy, an expert in medical record documentation, said collectively the reviews show that the problem is “absolutely endemic” in the industry.
Auditors are finding the same inflated charges “over and over again,” he said, adding: “I don’t think there is enough oversight.”
When it comes to getting money back from the health plans, extrapolation is the big sticking point.
Although extrapolation is routinely used as a tool in most Medicare audits, CMS officials have never applied it to Medicare Advantage audits because of fierce opposition from the insurance industry.
“While this data is more than a decade old, more recent research demonstrates Medicare Advantage’s affordability and responsible stewardship of Medicare dollars,” said Mary Beth Donahue, president of the Better Medicare Alliance, a group that advocates for Medicare Advantage. She said the industry “delivers better care and better outcomes” for patients.
But critics argue that CMS audits only a tiny percentage of Medicare Advantage contracts nationwide and should do more to protect tax dollars.
Doolittle, the former CMS official, said the agency needs to “start keeping up with the times and doing these audits on an annual basis and extrapolating the results.”
But Kathy Poppitt, a Texas health care attorney, questioned the fairness of demanding huge refunds from insurers so many years later. “The health plans are going to fight tooth and nail and not make this easy for CMS,” she said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
USE OUR CONTENT
This story can be republished for free (details).
2 years 8 months ago
Health Care Costs, Health Industry, Insurance, Medicare, CMS, Connecticut, Florida, Insurers, texas
Medical News, Health News Latest, Medical News Today - Medical Dialogues |
MCC Releases Reporting Schedule For Mop up Round NEET PG Counselling Candidates, Details
New Delhi : Through a recent notice, the Medical Counselling Committee (MCC) has released the reporting schedule for mop up round of NEET PG counselling.
Medical counselling committee had published provisional result of Mop Up Round NEET-PG 2022 Counselling on 19.11.2022 on MCC website, following which final Result is published on MCC website.
New Delhi : Through a recent notice, the Medical Counselling Committee (MCC) has released the reporting schedule for mop up round of NEET PG counselling.
Medical counselling committee had published provisional result of Mop Up Round NEET-PG 2022 Counselling on 19.11.2022 on MCC website, following which final Result is published on MCC website.
To view the final result, click here-- https://mcc.nic.in/WebinfoMedical/File/ViewFile?FileId=4761&LangId=P
All the allotted candidates are advised to strictly adhere the counselling schedule as mentioned below:
Allotment Letter for Mop Up Round is now available for Download and Reporting will start as per Notice.
Mop Up Round
Result
Reporting
19th November, 2022
20th November, 2022 to 24th November,
2022
(1-Day)
(05-Days)
All candidates shall ensure that admission process by allotted college should be made through online reporting portal of intraMCC. Any admission take through offline mode will be treated null & void.
To view the official Notice,Click here : https://medicaldialogues.in/pdf_upload/notice-for-reporting-of-mop-up-round-pg-counsellin-191557.pdf
MCC is conducting the counselling for the following Institutions/Universities:-
a) 50% All India Quota seats of all states including Union Territory of Jammu & Kashmir, this year onwards. (UT of J&K will also contribute their 50% broad speciality Medical/ Dental seats for the All India Quota counselling conducted by MCC of DGHS).b) 100% seats (All India Quota seats + Institutional Quota seats) of Central Universities (Aligarh Muslim University/ Banaras Hindu University/ University of Delhi/ Central Institutes as per eligibility conditions mentioned in Important Questions related to Scheme of Counselling duly uploaded on MCC website.c) 100% seats of Deemed Universities.d) 50% AIQ P.G seats of colleges under Employee State Insurance Corporation (wards of ESIC insured persons).e) All P.G seats of Armed Forces Medical Services Institutions(only Registration part).f) Central Institutes, VMMC & Safdarjung Hospital, ABVIMS & RML Hospital and ESIC Institute, PGIMSR, Basaidarapur(50% All India Quota seats and 50% seats of I.P University).
2 years 8 months ago
State News,News,Delhi,Medical Education,Medical Colleges News,Medical Courses News,Medical Admission News,Latest Medical Education News
Therapeutic treatment centre for children almost ready
THE therapeutic treatment centre where mental health interventions will be provided to the more than 4,600 Jamaican children in State care needing those services, is almost ready.
The centre, which is being constructed on lands located at Maxfield Park Children's Home in St Andrew, is "90-odd per cent complete" and should be open before the fiscal year ends in March, according to Rosalee Gage-Grey, head of the Child Protection and Family Services Agency (CPFSA) which will operate the entity.
Cabinet in May last year had given approval for the construction of the $117.1-million facility, the contract for which was awarded to Alfrasure Structures and Roofing Limited. The scope of work involved the building of a 650m2 model therapeutic care centre for children, "with a series of rooms for consultation, observation, operations and administration". This was to be constructed over six months according to the bid document seen by the Jamaica Observer.
"We are a little behind schedule because it should have been done in six months, but you know construction in this country. But, the building is up, they are doing some of the finishing work to the interior and, like, parking and those kinds of finishes. We are very anxious to have it open because it is very well needed," Gage-Grey expressed.
According to statistics, there are more than 800,000 children in the island's 14 parishes, nearly 120,000 of whom may have a mental disorder and with 40,000 suffering from a severe mental disorder. Child guidance clinics islandwide, of which there are 20, tend to 3,500 Jamaican children but experts believe that more than 95 per cent — or just over 110,000 children and adolescents with mental disorders — are slipping through the cracks and not benefiting from the government-provided services.
There are 30,000 children in the Kingston and St Andrew region alone who need psychosocial intervention. It would take at least another five to seven clinics to provide services to even two-thirds of them.
Gage-Grey said while the centre is predominantly for children in the care of the State, other children will be able to access the services.
In the meantime she said the centre will not be residential, as had been originally planned, however, children in crisis situations who need to be relocated will be given temporary housing on the grounds.
Gage-Grey was unable to say whether the project had been completed within budget.
Child and adolescent psychiatrist Dr Ganesh Shetty, at the onset, had described the plans for the facility as "a step in the right direction". He, however, told the Observer that based on the number of children in Jamaica suspected of suffering from mental health issues, one such facility per parish is what it will take to even make a dent in the situation.
Chief among his concerns are the children who are suffering in silence, their conditions undetected rendering them prime targets for gangs and criminal activities.
According to research, in the United States two out of three such children do not receive help, in Canada four out of five children do not get assistance, while in Jamaica 19 out of 20 children suffer the same fate.
2 years 8 months ago