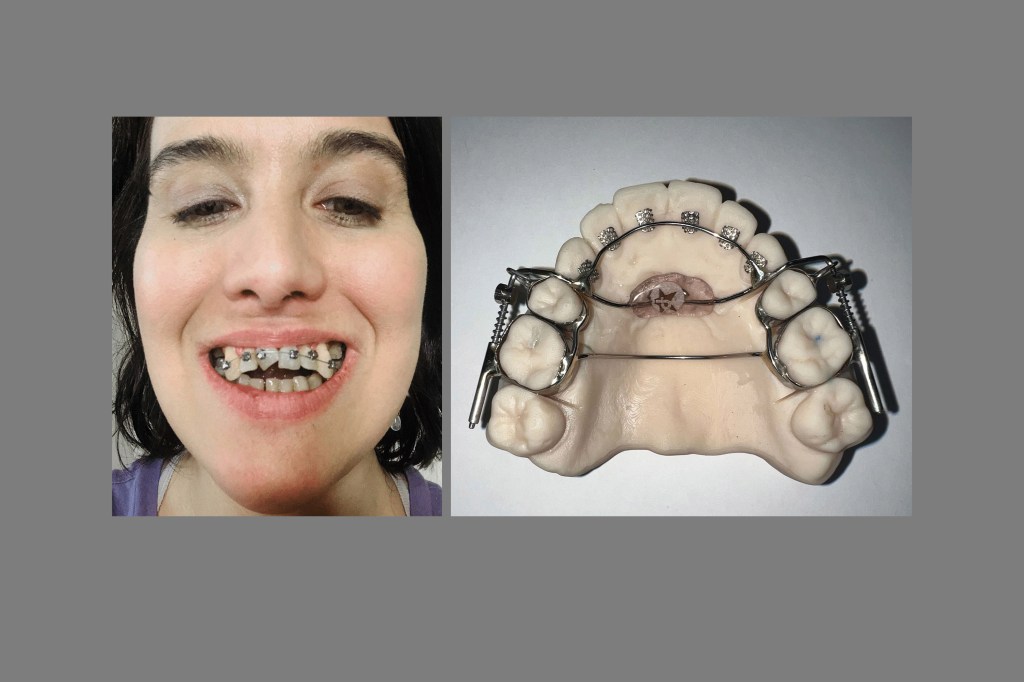
FDA Said It Never Inspected Dental Lab That Made Controversial AGGA Device
The FDA never inspected Johns Dental Laboratories during more than a decade in which it made the Anterior Growth Guidance Appliance, or “AGGA,” a dental device that has allegedly harmed patients and is now the subject of a criminal investigation.
According to FDA documents obtained through the Freedom of Information Act, the agency “became aware” of the AGGA from a joint investigation by KFF Health News and CBS News in March 2023, then responded with its first-ever inspection of Johns Dental months later.
That inspection found that the Indiana dental device manufacturer didn’t require all customer complaints to be investigated and the company did not investigate some complaints about people being hurt by products, including the AGGA, the FDA documents state. The FDA requires device companies to investigate complaints and forward them to the agency. Johns Dental had “never” alerted the FDA to any such complaints, according to the documents.
The AGGA, which its inventor testified has been used on more than 10,000 patients, was promoted by dentists nationwide, some of whom said it could “grow” or “expand” an adult’s jaw without surgery and treat common ailments like sleep apnea. But these claims were not backed by peer-reviewed research, and Johns Dental has settled lawsuits from 20 patients who alleged the AGGA caused them grievous harm. The company has not admitted liability.
Two former FDA officials said the AGGA was likely able to stay on the market — and off the FDA’s radar — for so long because of the lack of inspections and investigations at Johns Dental. Madris Kinard, a former FDA manager who founded Device Events, which analyzes FDA data, said it defies belief that Johns Dental never received a complaint worthy of relaying to the FDA.
“That’s a red flag for me. If I don’t see a single report to the FDA, I typically think there is something going on,” Kinard said. “When they don’t report, what you have is devices that stay on the market much longer than they should. And patients get harmed.”
Johns Dental Laboratories declined to comment when reached by phone and its lawyers did not respond to requests for an interview. The family-owned company, which has operated since 1939 in the western Indiana city of Terre Haute, sells dozens of products to dentists and makes hundreds of retainers and sleep apnea appliances each month, according to its website.
Twelve of Johns Dental’s products are registered with the FDA as Class II medical devices, meaning they carry at least a moderate risk, and some have been featured on the company website for at least two decades, according to screen captures preserved by the Internet Archive.
The AGGA, which was invented by Tennessee dentist Steve Galella in the 1990s, was not registered with the FDA like Johns Dental’s other devices. Company owner Jerry Neuenschwander has said in sworn court depositions that Johns Dental started making the AGGA in 2012 and became Galella’s exclusive manufacturer in 2015 and that at one point the AGGA was responsible for about one-sixth of Johns Dental’s total sales revenue.
In another deposition, Johns Dental CEO Lisa Bendixen said the company made about 3,000 to 4,000 AGGAs a year and paid Galella’s company a “royalty” of $50 to $65 for every sale.
“We are not dentists. We do not know how these appliances work. All we do is manufacture to Dr. Galella’s specifications,” she said, according to a deposition transcript.
The FDA’s lack of knowledge about the AGGA likely contributed to its loose oversight of Johns Dental. When asked to explain the lack of inspection, the FDA said that, based on what it knew at the time, it was not required to inspect Johns Dental until 2018 when the company registered as a “contract manufacturer” of other medical devices. Prior to 2018, the FDA was only aware of Johns Dental operating as a “dental laboratory,” which normally do not manufacture their own products and only modify devices made by other companies to fit dentists’ specifications. The FDA does not regularly inspect dental labs, although it can if it has concerns or gets complaints, the agency said.
Kinard said that based on her experience at the FDA she believes the agency prioritizes medical devices over dental devices, which may have contributed to the lack of inspections at Johns Dental.
“There hasn’t been much attention to dental devices in the past,” Kinard said. “Hopefully that’s going to change because of dental implant failures, as well as this device, which has quite obviously had serious issues.”
The AGGA resembles a retainer and uses springs to apply pressure to the front teeth and upper palate, according to a patent application. Last year, the KFF Health News-CBS News investigation revealed the AGGA was not backed by any peer-reviewed research and had never been submitted to the FDA for review. At the time, at least 20 patients had alleged in lawsuits that the AGGA had caused grievous harm to their teeth, gums, and bone — and some said they’d lost teeth. Multiple dental specialists said in interviews that they had examined AGGA patients whose teeth had been shoved out of position by the device, sometimes causing tens of thousands of dollars in damage.
“The entire concept of this device, of this treatment, makes zero sense,” said Kasey Li, a maxillofacial surgeon who published research on AGGA patients that appeared on a National Institutes of Health website. “It doesn’t grow the jaw. It doesn’t widen the jaw. It just pushes the teeth out of their original position.
Johns Dental and Galella have negotiated out-of-court settlements with the original 20 AGGA plaintiffs without publicly admitting fault. At least 13 more AGGA patients have filed similar lawsuits since the KFF Health News-CBS News investigation. Johns Dental and Galella denied wrongdoing or have not yet responded to the allegations in the newer lawsuits.
Galella declined to be interviewed in 2023 and neither he nor his attorneys responded to recent requests for comment. One of his attorneys, Alan Fumuso, said in a 2023 statement that the AGGA “is safe and can achieve beneficial results” when used properly.
In the wake of the KFF Health News-CBS News report, Johns Dental abruptly stopped making the AGGA, according to the newly released FDA documents. The Department of Justice soon after opened a criminal investigation into the AGGA that was ongoing as of December, according to court filings. No charges have been filed. A DOJ spokesperson declined comment.
Spurred by the March 2023 news report, the FDA inspected Johns Dental in July. The FDA’s website shows that Johns Dental was issued seven citations, but the substance of the agency’s findings was not known until the inspection report was obtained this year.
FDA investigator David Gasparovich wrote in that report that he arrived unannounced at Johns Dental last July and was met by five attorneys who instructed employees not to answer any questions about the AGGA or the company’s complaint policies. Neuenschwander was told by his attorney not to talk to the inspector, the report states.
“He asked if he could photograph my credentials,” Gasparovich wrote in his report. “This was the last conversation I would have with Mr. Neuenschwander at the request of his attorney.”
The FDA requires device companies to investigate product complaints and submit a “medical device report” to the agency within 30 days if the products may have contributed to serious injury or death. Gasparovich’s inspection report states that Johns Dental had “not adequately investigated customer complaints,” and its complaint policies were “not adequately established,” allowing employees to not investigate if the product was not first returned to the company.
Johns Dental received four complaints about the AGGA after the KFF Health News-CBS News report, including one that came after the FDA announced “safety concerns” about the device, according to the inspection report.
“Zero (0) out of the four (4) complaints were investigated,” Gasparovich wrote in the report. “Each complaint was closed on the same day it was received.”
In the months after Gasparovich’s inspection, Johns Dental sent letters to the FDA saying it revised its complaint policies to require more investigations and hired a consultant and an auditor to address other FDA concerns, according to the documents obtained through FOIA.
Former FDA analyst M. Jason Brooke, now an attorney who advises medical device companies, said the FDA uses an internal risk-based algorithm to determine when to inspect manufacturers and he advises his clients to expect inspections every three to five years.
Brooke said the AGGA is an example of how the FDA’s oversight can be hamstrung by its reliance on device manufacturers to be transparent. If device companies don’t report to the agency, it can be left unaware of patient complaints, malfunctions, or even entire products, he said.
When a company “doesn’t follow the law,” Brooke said, “the FDA is in the dark.”
“If there aren’t complaints coming from patients, doctors, competitors, or the company itself, then in a lot of ways, there’s just a dearth of information for the FDA to consume to trigger an inspection,” Brooke said.
CBS News producer Nicole Keller contributed to this article.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
USE OUR CONTENT
This story can be republished for free (details).
1 year 2 months ago
Health Industry, Dental Health, FDA, Indiana, Investigation, Tennessee, When Medical Devices Malfunction
La gripe aviar es mala para las aves de corral y las vacas lecheras. No es una amenaza grave para la mayoría de nosotros… por ahora
Los titulares explotaron después que el Departamento de Agricultura confirmara que el virus de la gripe aviar H5N1 ha infectado a vacas lecheras en todo el país.
Las pruebas han detectado el virus en el ganado en nueve estados, principalmente en Texas y Nuevo México, y más recientemente en Colorado, dijo Nirav Shah, director principal adjunto de los Centros para el Control y Prevención de Enfermedades (CDC), en un evento del 1 de mayo.
Otros animales, y al menos una persona en Texas, también se infectaron con el H5N1. Pero lo que más temen los científicos es si el virus se propagara de manera eficiente de persona a persona. Eso no ha sucedido y podría no suceder. Shah dijo que los CDC consideran que el brote de H5N1 “es un riesgo bajo para el público en general en este momento”.
Los virus evolucionan y los brotes pueden cambiar rápidamente. “Como con cualquier brote importante, esto se mueve a la velocidad de un tren bala”, dijo Shah. “De lo que hablamos ahora es de un instantánea de ese tren que se mueve rápidamente”. Lo que quiere decir es que lo que hoy se sabe sobre la gripe aviar H5N1 seguramente cambiará.
Con eso en mente, KFF Health News explica lo que se necesita saber ahora.
¿Quién contrae el virus que causa la gripe aviar?
Principalmente las aves. Sin embargo, en los últimos años, el virus de la gripe aviar H5N1 ha estado saltando cada vez más de las aves a los mamíferos en todo el mundo. La creciente lista, de más de 50 especies, incluye focas, cabras, zorrinos, gatos y perros salvajes en un zoológico en el Reino Unido. Al menos 24,000 leones marinos murieron en brotes de gripe aviar H5N1 en Sudamérica el año pasado.
Lo que hace que el brote actual en el ganado sea inusual es que se está propagando rápidamente de vaca a vaca, mientras que los otros casos, excepto las infecciones de leones marinos, parecen limitados. Los investigadores saben esto porque las secuencias genéticas de los virus H5N1 extraídos de las vacas este año eran casi idénticas entre sí.
El brote de ganado también preocupa porque agarró al país desprevenido. Los investigadores que examinan los genomas del virus sugieren que originalmente se transmitió de las aves a las vacas a finales del año pasado en Texas, y desde entonces se ha propagado entre muchas más vacas de las que se han examinado.
“Nuestros análisis muestran que esto ha estado circulando en vacas durante unos cuatro meses, bajo nuestras narices”, dijo Michael Worobey, biólogo especializado en evolución de la Universidad de Arizona en Tucson.
¿Es este el comienzo de la próxima pandemia?
Aún no. Pero es algo que vale la pena considerar porque una pandemia de gripe aviar sería una pesadilla. Más de la mitad de las personas infectadas por cepas anteriores del virus de la gripe aviar H5N1 de 2003 a 2016 murieron.
Incluso si las tasas de mortalidad resultan ser menos severas para la cepa H5N1 que circula actualmente en el ganado, las repercusiones podrían implicar muchas personas enfermas y hospitales demasiado abrumados para manejar otras emergencias médicas.
Aunque al menos una persona se infectó con el H5N1 este año, el virus no puede provocar una pandemia en su estado actual.
Para alcanzar este horrible estatus, un patógeno necesita enfermar a muchas personas en varios continentes. Y para lograrlo, el virus H5N1 necesitaría infectar a toneladas de personas. Eso no sucederá a través de saltos ocasionales del virus de los animales de granja a las personas. Más bien, el virus debe adquirir mutaciones para propagarse de persona a persona, como la gripe estacional, como una infección respiratoria transmitida principalmente por el aire cuando las personas tosen, estornudan y respiran.
Como aprendimos de covid-19, los virus transmitidos por el aire son difíciles de frenar.
Eso aún no ha sucedido. Sin embargo, los virus H5N1 ahora tienen muchas oportunidades para evolucionar a medida que se replican dentro de los organismos de miles de vacas. Como todos los virus, mutan a medida que se replican, y las mutaciones que mejoran la supervivencia del virus se transmiten a la próxima generación. Y debido a que las vacas son mamíferos, los virus podrían estar mejorando en reproducirse dentro de células más cercanas a las nuestras que las de las aves.
La evolución de un virus de gripe aviar listo para una pandemia podría facilitarse por una especie de superpoder que poseen muchos virus. Es decir, a veces intercambian sus genes con otras cepas en un proceso llamado recombinación.
En un estudio publicado en 2009, Worobey y otros investigadores rastrearon el origen de la pandemia del virus de la gripe porcina H1N1 en eventos en los que diferentes virus que causaban esta gripe, la gripe aviar y la gripe humana mezclaban y combinaban sus genes dentro de cerdos que se estaban infectando simultáneamente. Los cerdos no necesitan estar involucrados esta vez, advirtió Worobey.
¿Comenzará una pandemia si una persona bebe leche contaminada con el virus?
Aún no. La leche de vaca, así como la leche en polvo y la fórmula infantil, que se venden en tiendas se consideran seguras porque la ley requiere que toda la leche vendida comercialmente sea pasteurizada. Este proceso de calentar la leche a altas temperaturas mata bacterias, virus y otros microorganismos.
Las pruebas han identificado fragmentos de virus H5N1 en la leche comercial, pero confirman que los fragmentos del virus están muertos y, por lo tanto, son inofensivos.
Sin embargo, la leche “cruda” no pasteurizada ha demostrado contener virus H5N1 vivos, por eso la Administración de Drogas y Alimentos (FDA) y otras autoridades sanitarias recomiendan firmemente a las personas que no la tomen, porque podrían enfermarse de gravedad o algo peor.
Pero, aún así, es poco probable que se desate una pandemia porque el virus, en su forma actual, no se propaga eficientemente de persona a persona, como lo hace, por ejemplo, la gripe estacional.
¿Qué se debe hacer?
¡Mucho! Debido a la falta de vigilancia, el Departamento de Agricultura (USDA) y otras agencias han permitido que la gripe aviar H5N1 se propague en el ganado, sin ser detectada. Para hacerse cargo de la situación, el USDA recientemente ordenó que se sometan a pruebas a todas las vacas lecheras en lactancia antes que los ganaderos las trasladen a otros estados, y que se informen los resultados de las pruebas.
Pero al igual que restringir las pruebas de covid a los viajeros internacionales a principios de 2020 permitió que el coronavirus se propagara sin ser detectado, testear solo a las vacas que se mueven entre estados dejaría pasar muchos casos.
Estas pruebas limitadas no revelarán cómo se está propagando el virus entre el ganado, información que los ganaderos necesitan desesperadamente para frenarlo. Una hipótesis principal es que los virus se están transfiriendo de una vaca a la siguiente a través de las máquinas utilizadas para ordeñarlas.
Para aumentar las pruebas, Fred Gingrich, director ejecutivo de la American Association of Bovine Practitioners, dijo que el gobierno debería ofrecer fondos a los ganaderos para que informen casos y así tengan un incentivo para hacer pruebas. De lo contrario, dijo, informar solo daña la reputación por encima de las pérdidas financieras.
“Estos brotes tienen un impacto económico significativo”, dijo Gingrich. “Los ganaderos pierden aproximadamente el 20% de su producción de leche en un brote porque los animales dejan de comer, producen menos leche, y parte de esa leche es anormal y no se puede vender”.
Gingrich agregó que el gobierno ha hecho gratuitas las pruebas de H5N1 para los ganaderos, pero no han presupuestado dinero para los veterinarios que deben tomar muestras de las vacas, transportar las muestras y presentar los documentos. “Las pruebas son la parte menos costosa”, explicó.
Si las pruebas en las granjas siguen siendo esquivas, los virólogos aún pueden aprender mucho analizando secuencias genómicas del virus H5N1 de muestras de ganado. Las diferencias entre las secuencias cuentan una historia sobre dónde y cuándo comenzó el brote actual, el camino que recorre y si los virus están adquiriendo mutaciones que representan una amenaza para las personas.
Sin embargo, esta investigación vital se ha visto obstaculizada porque el USDA publica los datos incompletos y con cuentagotas, dijo Worobey.
El gobierno también debería ayudar a los criadores de aves de corral a prevenir brotes de H5N1, ya que estos matan a muchas aves y representan una amenaza constante de potenciales saltos de especies, dijo Maurice Pitesky, especialista en enfermedades de aves de la Universidad de California-Davis.
Las aves acuáticas como los patos y los gansos son las fuentes habituales de brotes en granjas avícolas, y los investigadores pueden detectar su proximidad mediante el uso de sensores remotos y otras tecnologías. Eso puede significar una vigilancia rutinaria para detectar signos tempranos de infecciones en aves de corral, usar cañones de agua para ahuyentar a las bandadas migratorias, reubicar animales de granja o llevarlos temporalmente a cobertizos. “Deberíamos estar invirtiendo en prevención”, dijo Pitesky.
Bien, no es una pandemia, pero ¿qué podría pasarle a las personas que contraigan la gripe aviar H5N1 de este año?
Realmente nadie lo sabe. Solo una persona en Texas fue diagnosticada con la enfermedad este año, en abril. Esta persona trabajaba con vacas lecheras, y tuvo un caso leve con una infección en el ojo. Los CDC se enteraron de esto debido a su proceso de vigilancia. Las clínicas deben alertar a los departamentos de salud estatales cuando diagnostican a trabajadores agrícolas con gripe, utilizando pruebas que detectan virus de la influenza en general.
Los departamentos de salud estatales luego confirman la prueba y, si es positiva, envían una muestra de la persona a un laboratorio de los CDC, donde se verifica específicamente la presencia del virus H5N1. “Hasta ahora hemos recibido 23”, dijo Shah. “Todos menos uno resultaron negativos”.
Agregó que funcionarios del departamento de salud estatal también están monitoreando a alrededor de 150 personas que han pasado tiempo alrededor de ganado. Están en contacto con estos trabajadores agrícolas con llamadas telefónicas, mensajes de texto o visitas en persona para ver si desarrollan síntomas. Y si eso sucede, les harán pruebas.
Otra forma de evaluar a los trabajadores agrícolas sería testear su sangre en busca de anticuerpos contra el virus de la gripe aviar H5N1; un resultado positivo indicaría que podrían haberse infectado sin saberlo. Pero Shah dijo que los funcionarios de salud aún no están haciendo este trabajo.
“El hecho de que hayan pasado cuatro meses y aún no hayamos hecho esto no es una buena señal”, dijo Worobey. “No estoy muy preocupado por una pandemia en este momento, pero deberíamos comenzar a actuar como si no quisiéramos que sucediera”.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
USE OUR CONTENT
This story can be republished for free (details).
1 year 2 months ago
Health Industry, Noticias En Español, Public Health, Rural Health, Colorado, FDA, Food Safety, New Mexico, texas
KFF Health News' 'What the Health?': Abortion Access Changing Again in Florida and Arizona
The Host
Julie Rovner
KFF Health News
Julie Rovner is chief Washington correspondent and host of KFF Health News’ weekly health policy news podcast, “What the Health?” A noted expert on health policy issues, Julie is the author of the critically praised reference book “Health Care Politics and Policy A to Z,” now in its third edition.
The national abortion landscape was shaken again this week as Florida’s six-week abortion ban took effect. That leaves North Carolina and Virginia as the lone Southern states where abortion remains widely available. Clinics in those states already were overflowing with patients from across the region.
Meanwhile, in a wide-ranging interview with Time magazine, former President Donald Trump took credit for appointing the Supreme Court justices who overturned Roe v. Wade, but he steadfastly refused to say what he might do on the abortion issue if he is returned to office.
This week’s panelists are Julie Rovner of KFF Health News, Sarah Karlin-Smith of the Pink Sheet, Alice Miranda Ollstein of Politico, and Rachana Pradhan of KFF Health News.
Panelists
Sarah Karlin-Smith
Pink Sheet
Alice Miranda Ollstein
Politico
Rachana Pradhan
KFF Health News
Among the takeaways from this week’s episode:
- Florida’s new, six-week abortion ban is a big deal for the entire South, as the state had been an abortion haven for patients as other states cut access to the procedure. Some clinics in North Carolina and southern Virginia are considering expansions to their waiting and recovery rooms to accommodate patients who now must travel there for care. This also means, though, that those traveling patients could make waits even longer for local patients, including many who rely on the clinics for non-abortion services.
- Passage of a bill to repeal Arizona’s near-total abortion ban nonetheless leaves the state’s patients and providers with plenty of uncertainty — including whether the ban will temporarily take effect anyway. Plus, voters in Arizona, as well as those in Florida, will have an opportunity in November to weigh in on whether the procedure should be available in their state.
- The FDA’s decision that laboratory-developed tests must be subject to the same regulatory scrutiny as medical devices comes as the tests have become more prevalent — and as concerns have grown amid high-profile examples of problems occurring because they evaded federal review. (See: Theranos.) There’s a reasonable chance the FDA will be sued over whether it has the authority to make these changes without congressional action.
- Also, the Biden administration has quietly decided to shelve a potential ban on menthol cigarettes. The issue raised tensions over its links between health and criminal justice, and it ultimately appears to have run into electoral-year headwinds that prompted the administration to put it aside rather than risk alienating Black voters.
- In drug news, the Federal Trade Commission is challenging what it sees as “junk” patents that make it tougher for generics to come to market, and another court ruling delivers bad news for the pharmaceutical industry’s fight against Medicare drug negotiations.
Plus, for “extra credit” the panelists suggest health policy stories they read this week that they think you should read, too:
Julie Rovner: ProPublica’s “A Doctor at Cigna Said Her Bosses Pressured Her To Review Patients’ Cases Too Quickly. Cigna Threatened To Fire Her,” by Patrick Rucker, The Capitol Forum, and David Armstrong, ProPublica.
Alice Miranda Ollstein: The Associated Press’ “Dozens of Deaths Reveal Risks of Injecting Sedatives Into People Restrained by Police,” by Ryan J. Foley, Carla K. Johnson, and Shelby Lum.
Sarah Karlin-Smith: The Atlantic’s “America’s Infectious-Disease Barometer Is Off,” by Katherine J. Wu.
Rachana Pradhan: The Wall Street Journal’s “Millions of American Kids Are Caregivers Now: ‘The Hardest Part Is That I’m Only 17,” by Clare Ansberry.
Also mentioned on this week’s podcast:
- Time’s “How Far Trump Would Go,” by Eric Cortellessa.
- NPR’s “Why Is a 6-Week Abortion Ban Nearly a Total Ban? It’s About How We Date a Pregnancy,” by Selena Simmons-Duffin.
- NPR’s “’Sicko’s’ Peeno Sees Few Gains in Health Insurance,” by Julie Rovner.
- CNN’s “Walmart Will Close All of Its Health Care Clinics,” by Nathaniel Meyersohn.
Click to open the Transcript
Transcript: Abortion Access Changing Again in Florida and Arizona
[Editor’s note: This transcript was generated using both transcription software and a human’s light touch. It has been edited for style and clarity.]
Mila Atmos: The future of America is in your hands. This is not a movie trailer, and it’s not a political ad, but it is a call-to-action. I’m Mila Atmos, and I’m passionate about unlocking the power of everyday citizens. On our podcast “Future Hindsight,” we take big ideas about civic life and democracy, and turn them into action items for you and me. Every Thursday, we talk to bold activists and civic innovators to help you understand your power and your power to change the status quo. Find us at futurehindsight.com or wherever you listen to podcasts.
Julie Rovner: Hello, and welcome back to “What the Health?” I’m Julie Rovner, chief Washington correspondent for KFF Health News, and I’m joined by some of the best and smartest health reporters in Washington. We’re taping this week on Thursday, May 2, at 10 a.m. As always, news happens fast and things might have changed by the time you hear this. So here we go.
We are joined today via video conference by Alice Miranda Ollstein of Politico.
Alice Miranda Ollstein: Hello.
Rovner: Sarah Karlin-Smith of the Pink Sheet.
Sarah Karlin-Smith: Hi, everybody.
Rovner: And my KFF Health News colleague Rachana Pradhan.
Rachana Pradhan: Hello.
Rovner: No interview this week, but more than enough news to make up for it, so we will dig right in. We will start, again, with abortion. On Wednesday, Florida’s six-week abortion ban took effect. Alice, what does this mean for people seeking abortions in Florida, and what’s the spillover to other states?
Ollstein: Yeah, this is a really huge deal not only because Florida is so populous, but because Florida, somewhat ironically given its leadership, has been a real abortion haven since Roe vs. Wade was overturned. A lot of its surrounding states had near-total bans go into effect right away. Florida has had a 15-week ban for a while, but that has still allowed for a lot of abortions to take place, and so a lot of people have been coming to Florida from Alabama, Louisiana, those surrounding states for abortions. Now, Florida’s six-week ban is taking effect and that means that a lot of the patients that had been going there will now need to go elsewhere, and a lot of Floridians will have to travel out of state.
And so there are concerns about whether the closest clinics they can get to, in North Carolina and southern Virginia, will have the capacity to handle that patient overload. I talked to some clinics that are trying to staff up. They’re even thinking about physical changes to their clinics, like building bigger waiting rooms and recovery rooms. This is going to cause a real crunch, in terms of health care provision. That is set to not only affect abortion, but with these clinics overwhelmed, that takes up appointments for people seeking other services as well. My colleagues and I have been talking to people in the sending states, like Alabama, who worry that the low-income patients they serve who were barely able to make it to Florida will not be able to make it even further. Then, we’ve talked to providers in the receiving states, like Virginia, who are worried that there just are simply not enough appointments to handle the tens of thousands of people who had been getting abortions in Florida up to this point.
Rovner: Of course, what ends up happening is that, if people have to wait longer, it pushes those abortions into later types of abortions, which are more complicated and more dangerous and more expensive.
Ollstein: Yes. While the rate of complication is low, the later in pregnancy you go, it does get higher. That’s another consideration as well.
I will flag, though, that restrictions on abortion pills in North Carolina, which is now one of the states set to receive a lot of people, those did get a little bit loosened by a court ruling this week so people will not have to have a mandatory in-person follow-up appointment for abortion pills like they used to have to have. That could help some patients who are traveling in from out of state, but a lot of restrictions remain, and it’ll be tough for a lot of folks to navigate.
Rovner: While we think of that, well there’s at least, you can get abortions up to six weeks, my friend Selena Simmons-Duffin over at NPR had a really good explainer about why six weeks isn’t really six weeks, because of the way that we measure pregnancy, that six weeks is really two weeks. It really is a very, very small window in which people will be able to get abortions in Florida. It’s not quite a full ban, but it is quite close to it.
Well, speaking of full bans, after several false starts, the Arizona Senate Wednesday voted to repeal the 1864 abortion ban that its Supreme Court ruled could take effect. The Democratic governor is expected to sign it. Where does that leave abortion law in the very swing state of Arizona? It’s kind of a muddle, isn’t it?
Ollstein: It is. The basics are that a 15-week ban is already in place and will continue to be in place once this repeal takes effect. What we don’t know is whether the total ban from before Arizona was even a state will take effect temporarily, because of the weird timing of the court’s implementation of that old ban, and the new repeal bill that just passed that the governor is expected to sign very soon. The total ban could go into effect, at least for a little bit over the summer. Planned Parenthood is positioning the court to not let that happen, to stay the implementation until the repeal bill can take effect. All of this is very much in flux. Of course, as we’ve seen in so many states, that leads to patients and providers just being very scared, and not knowing what’s legal and what’s not, and folks being unable to access care that may, in fact, be legal because of that. Of course, this is all in the context of Arizona, as well as Florida, being poised to vote directly on abortion access this fall. If the total ban does go into effect temporarily, it’s sure to pour fuel on that fire and really rile people up ahead of that vote.
Rovner: Yeah, I was going to mention that. Well, now that we’re talking about politics. This week, we heard a little bit more about how former President Trump wants to handle the abortion issue, via a long sit-down interview with Time magazine. I will link to that interview in the show notes. The biggest “news” he made was to suggest that he’d have an announcement soon about his views on the abortion pill. But he said that would come in the next two weeks, the interview was of course more than two weeks ago. They did a follow-up two weeks later and he still said it was coming. In the follow-up interview, he said it would be next week, which this has already passed. Do we really expect Trump to say something about this, or was that just him deflecting, as we know he is wont to do?
Pradhan: Well, I’m sure that he’s getting pressure to say something, because as people have noted now quite widely, regardless of individual state laws, there are certainly conservatives that are pushing for him and his future administration to ban the mailing of abortion pills using the Comstock Act from the 1800s, which would basically annihilate access to that form of terminating pregnancies.
Rovner: There are also some who want him to just repeal the FDA approval, right?
Ollstein: Right. Of course, the Biden administration has made it easier for folks to get access to those, to mifepristone, in particular, one of two pills that are used in medication abortion. But yeah, will it be two weeks? I think he obviously knows that this is a potential political liability for him, so whether he’ll say something, I’m sure he will get competing advice as to whether it’s a good idea to say something at all, so we’ll have to see.
Rovner: Well, speaking of Trump deflecting, he seemed to be pretty disciplined about the rest of the abortion questions — and there were a lot of abortion questions in that interview — insisting that, while he takes credit for appointing the justices who made the majority to overturn Roe, everything else is now up to the state. But by refusing to oppose some pretty-out-there suggestions of what states might do, Trump has now opened himself up to apparently accepting some fairly unpopular things, like tracking women’s menstrual periods. Lest you think that’s an overstatement, the Missouri state health director testified at a hearing last week that he kept a spreadsheet to track the periods of women who went to Planned Parenthood, which, according to The Kansas City Star, “helped to identify patients who had undergone failed abortions.” Yet, none of these things ever seem to stick to Trump. Is any of this going to matter in the long run? He’s clearly trying to walk this line between not angering his very anti-abortion base and not seeming to side too much with them, lest he anger a majority of the rest of the people he needs to vote for him.
Ollstein: Well, he’s also not been consistent in saying it’s totally up to states, whatever states want to do is fine. He’s repeatedly criticized Florida’s six-week ban. He refused to say how he would vote on the referendum to override it. He has criticized the Arizona ruling to implement the 1864 ban. This isn’t a pure “whatever states do is fine” stance, this is “whatever states do, unless it’s something really unpopular, in which case I oppose it.” That is a tough line to walk. The Biden administration and the Biden campaign have really seized on this and are trying to say, “OK, if you are going to have a leave-it-to-states stance, then we’re going to try to hang on you every single thing states do, whether it’s the legislature, or a court, or whatever, and say you own all of this.” That’s what’s playing out right now.
Rovner: I highly recommend reading the interview, because the interviewer was very skilled at trying to pin him down. He was pretty skilled at trying to evade being pinned down. Well, meanwhile, Republican attorneys general from 17 states are suing the Equal Employment Opportunity Commission from including abortion in a list of conditions that employers can’t discriminate against and must provide accommodations for, under rules implementing the Pregnant Workers Fairness Act. The new rules don’t require anyone to pay for anything, but they could require employers to provide leave or other accommodations to people seeking pregnancy-related health care. The EEOC has included abortion as pregnancy-related health care. This is yet another case that we could see making its way to the Supreme Court. Ironically, the Pregnant Workers Fairness Act was a very bipartisan bill, so there are a lot of anti-abortion groups that are extremely angry that this has been included in the regulation. This is one of those abortion-adjacent issues that tends to drag abortion in, even when it was never expected to be there. And we’re going to see more of these. We’re going to get back into the spending bills, as Congress tries to muddle its way through another session.
Pradhan: I think, when I think about this, even though there’s a regulatory battle and a legal one now, too, like in the immediate aftermath of the Dobbs [v. Jackson Women’s Health Organization] decision, when there were employers, I think about it more practically. Which is that there were employers that were saying, “We would cover expenses.” Or they would pay for people to travel out of state if that was something that they needed. I wonder how many people would actually do it, even if it exists, because that’s a whole other … Getting an abortion, or even things related to pregnancy, are incredibly private things, so I don’t know how many women would be willing to stand up and say, “Hey, I need this accommodation and you have to give it to me under federal regulations.” In a way, I think it’s notable both that the EEOC put out those regulations and that there’s litigation over it, but I wonder if it, practically speaking, just how much of an impact it would really have, just because of those privacy and practical hurdles associated with divulging information in that regard.
Rovner: As we were just talking about, somebody in Alabama, the closest place they can go to get an abortion is in North Carolina or Virginia, and go, “Hey, I need three days off so I can drive halfway across the country to get an abortion because I can’t get one here.” I see that might be an awkward conversation.
Pradhan: Just like any sensitive medical- or health-related needs, it’s not like people are rushing to tell their employers necessarily that it’s something that they’re dealing with.
Rovner: That’s true. It doesn’t have anything to do with privacy. Most people are not anxious to advertise any health-related issues that they are having. Speaking of people and their sensitive medical information, that Change Healthcare hack that we’ve been talking about since February, well the CEO of Change’s owner, UnitedHealth Group, was on Capitol Hill on Wednesday, taking incoming from both the Senate Finance Committee in the morning, and the House Energy and Commerce Committee in the afternoon. Among the other things that Andrew Witty told lawmakers was that the portal that was hacked did not have multifactor authentication and he confirmed that United paid $22 million in bitcoin to the hackers, although as we discussed last week, they might not have paid the hackers who actually had possession of the information. Nobody actually seemed to follow up on that, which I found curious. My favorite moment in the Senate hearing was when North Carolina Republican Thom Tillis offered CEO Witty a copy of the book “Hacking For Dummies.” Is anything going to result from these hearings? Other than what it seemed a lot of lawmakers getting to express their frustration in person.
Pradhan: Can I just say how incredible it is to me that a company that their net worth is almost $450 billion, one of the largest companies in the world, apparently does not know how to enforce rules on two-factor authentication, which is something I think that is very routine and commonplace among the modern industrialized workforce.
Rovner: I have it for my Facebook account!
Pradhan: Right. I think everyone, even in our newsroom, knows how to do it or has been told that this is necessary for so many things. I just find it absolutely unbelievable that the CEO of United would go to senators and say this, and think that it would be well-received, which it was not.
Rovner: I will say his body language seemed to be very apologetic. He didn’t come in guns blazing. He definitely came in thinking that, “Oh, I’m going to get kicked around, and I’m just going to have to smile and take it.” But obviously, this is still a really serious thing and a lot of members of Congress, a lot of the senators and the House members, said they’re still hearing from providers who still can’t get their claims processed, and from people who can’t get their medications because pharmacies can’t process the claims. There’s a lot of dispute about how long it’s going to take to get things back up and running. One of the interesting tidbits that I took away is that, as much of health care that goes through Change, it’s like 40% of all claims, it’s actually a minimum part of United’s health claims. United doesn’t use Change for most of its claims, which surprised me. Which is maybe why United isn’t quite as freaked out about this as a lot of others are. Is there anything Congress is going to be able to do here, other than say to their constituents, “Hey, I took your complaints right to the CEO?”
Karlin-Smith: I think there’s two things they may focus on. One is just cybersecurity risks in health care, which is broader than just these incidents. In some ways, it could be much worse, if you think about hospitals and medical equipment being hacked where there could be direct patient impacts in care because of it. The other thing is, United is such a large company and the amount of Americans impacted by this, but also the amount of different parts of health care they have expanded into, is really under scrutiny. I think it’s going to bring a light onto how big they’ve become, the amount of vertical integration in our health system, and the risks from that.
Rovner: We went through this in the ’90s. Vertical integration would make things more efficient, because everybody would have what they called aligned incentives, everybody would be working towards the same goal. Instead, we’ve seen that vertical integration has just created big, behemoth companies like United. I don’t know whether Congress will get into all of that, but at least it brought it up into their faces.
There’s lots of regulatory news this week. I want to start with the FDA, which finalized a rule basically making laboratory-developed tests medical devices that would require FDA review. Sarah, this has been a really controversial topic. What does this rule mean and why has there been such a big fight?
Karlin-Smith: This rule means that diagnostic tests that are developed, manufactured, and then actually get processed, and the results get processed at the lab, will now no longer be exempted from FDA’s medical device regulations and they’ll have to go through the process of medical devices. The idea is to basically have more oversight over them, to ensure that these tests are actually doing what they’re supposed to do, you’re getting the right results and so forth. Initially, over the years, the prevalence of these tests has grown, and what they’re used for, I think, has changed and developed where FDA is more concerned about the safety and the types of health decisions people may be making without proper oversight of the tests. One, I think, really infamous example that maybe can people use to understand this is Theranos was a company that was exempted from a lot of regulations because of being considered an LDT. The initial impact is going to be interesting because they’re actually basically exempting all already-on-the-market products. There’s also going to be some other exemptions, such as for tests that meet an unmet medical need, so I think that will have to be defined. There is a reasonable chance that there’s going to be lawsuits challenging whether FDA can do this on their own or need Congress to write new legislation. There have been battles over the years for Congress to do that. FDA, I think, has finally gotten tired of waiting for them to lead. I think initially, we’re going to see a lot of battles going forth and FDA also just has limited capacity to review some of this stuff.
Rovner: We already know that FDA has limited capacity on the medical device side. I was amused to see, oh, we’re going to make these medical devices, where there’s already a huge problem with FDA either exempting things that shouldn’t really be exempt, or just not being able to look at everything they should be looking at.
Karlin-Smith: Right. They’re going to take what they call a risk-based approach, which is a common terminology used at the FDA, I think, to focus on the things where they think there’s the most risk of something problematic happening to people’s health and safety if something goes wrong. It’s also an admission, to some extent, of something that’s not necessarily their fault, which is they only have so much budget and so many people, and that really comes down to Congress deciding they want to fix it. Now, FDA has user-fee programs and so forth, so perhaps they could convince the industry to pony up more money. But as you alluded to early on, one of the fights over this has been their different segments of companies that make these tests that have different feelings about the regulations. Because you have more traditional, medical device makers that are used to dealing with the FDA that probably feel like they have this leg up, they know how to handle a regulatory agency like FDA and get through. Then you have other companies that are smaller, and do not have that expertise, maybe don’t feel like they have the manpower and, just, money to deal with FDA. I think that’s where you get into some of these business fights that have also kept this on the sidelines for a while.
Pradhan: Well, also I wonder, hospitals also use laboratory developed tests, too, and they develop them. I feel like, and Sarah, correct me if I’m wrong, but I think previously when there was debate over whether FDA was going to do this, I think hospitals were pretty critical of any move of FDA to start regulating these more aggressively, right? Because they said for tests used for cancer detection or other health issues, I think that they were not thrilled at the idea. I don’t know that they’ve had to really deal with FDA in this regard either when it comes to devices.
Karlin-Smith: Yeah. I know one big exemption that people were looking for was whether they were going to exempt academic medical centers, and they did not. We’ll see what happens with that moving forward. But obviously, again, the older ones will have this exemption.
Rovner: Well, speaking of controversial regulations, the administration has basically decided that it’s not going to decide about the potential menthol ban that we’ve been talking about on and off. There was a statement from HHS [Department of Health and Human Services] last week that just said, “We need to look at this more.” Somebody remind us why this is so controversial. Obviously, health interests say, really, we should ban menthol, it helps a lot of people to continue smoking and it’s not good for health. Why would the administration not want to ban menthol?
Pradhan: It’s controversial because, I’ll just say, that it’s an election year and they are worried about backlash from Black voters not supporting President Biden in his reelection campaign, because they do this.
Karlin-Smith: It’s a health versus criminal justice issue, because the concern is that yes, in theory, if Black people make up the majority of people who use menthol cigarettes, you’re obviously protecting their health by not having it. But the concern has been among how this would be enforced in practice and whether it would lead to overpolicing of Black communities and people being charged or facing some kind of police brutality for what a lot of people would consider a minor crime. That’s where the tension has been. Although notably, some groups like the NAACP and stuff have been gotten on board with banning menthol. It’s an interesting thing where we’re trying to solve a policing or criminal justice problem through a health problem, rather than just solving the policing problem.
Ollstein: Like Sarah said, you have civil rights groups lined up on both sides of this fight. You have some saying that banning menthol cigarettes would be racist because they’re predominantly used by the Black population. But then you have people saying, well it’s racist to continue letting their health be harmed, and pointing out that those flavored cigarettes have been targeted in their marketing towards Black consumers, and that being a racist legacy that’s been around for a while. There’s these accusations on both sides and it seems like the politics of it are scaring the administration away a little bit.
Rovner: Well, just speaking of things that are political and that people smoke, the Drug Enforcement Administration announced its plan to downgrade the classification of marijuana, which until now has been included in the category of most dangerous drugs, like heroin and LSD, to what’s called Schedule III, which includes drugs with medicinal use that can also be abused, like Tylenol with codeine. But apparently, it could be awhile before it takes effect. This may not happen in time for this year’s election, right?
Karlin-Smith: Right. They have to release a proposed rule, you got to do comments, you got to get to the final rule. OMB [Office of Management and Budget] even. It’s supposedly at OMB now. OMB could hold it up for a while if they want to. As anybody who follows health policy in [Washington] D.C. knows, nothing moves fast here when it comes to regulations.
Rovner: Yes. A regulation that we thought was taken care of, but that actually only came out last week would protect LGBTQ+ Americans from discrimination in health care settings. This was a provision of the Affordable Care Act that the Trump administration had reversed. The Biden administration announced in 2021 that it wouldn’t enforce the Trump rules. But this is still a live issue in many courts and it’s significant to have these final regulations back on the books, yes?
Pradhan: It is. I think this is one of the ACA regulations that has ping-ponged the most, ever since the law was passed, because there have been lawsuits. I want to say it took the Obama administration years to even issue the first one, I think knowing how controversial it was. I believe it was the second, I think it was his second term and it was when there was no fear of repercussions for his reelection. Yeah, it’s been a very, very long-fought battle and I imagine this is also not the end of it. But no, it is very significant, the way that they defined the regulations.
Rovner: I confess, I was surprised when they came out because I thought it had already happened. I’m like, “Oh, we were still kicking this around.” So, now they appear to be final.
Well, finally this week, lots of news in health business. First, an update from last week. The Federal Trade Commission is challenging so-called junk patents from some pretty blockbuster drugs, charging that the patents are unfairly blocking generic competition. Sarah, what is this and why does it matter?
Karlin-Smith: FDA has what’s known as an orange book, as a part of a very complicated process set up by the 1984, I believe, Hatch-Waxman Act that was a compromise between the brand and the generic drug industries to get generic drugs to market a bit faster. FTC has been accusing companies of improperly listing patents in the orange book that shouldn’t be there, and thus making it harder to get generic products on the market. In particular, they’ve been actually going against drugs that have a device component, basically saying these components’ patents are not supposed to be in the orange book. They are basically asking the companies to delist the patents. They actually have gotten some concessions so far, from some of the other products they’ve targeted.
The idea would be this should help speed some of the generic entrants. It’s not quite as simple, because you do have lots of patents covering these drugs, so it does make it a little bit easier, but it’s not like it automatically opens the door. But it is unique and interesting that they have focused in on these targets because, typically, what are sometimes known as complex generics, are a lot harder for companies to make and get into the market because of the devices. Because for safety reasons FDA wants the devices to be very similar. If you pick up your product at the pharmacy, you have to be able to just know how to use it, really, without thinking about it, even if it’s a …
Rovner: Obviously, this covers things like inhalers and injectables.
Karlin-Smith: Right. The new weight loss drugs everybody is focused on, inhalers has been a big one as well. Things like an EpiPen, or stuff like that.
I think it’s been interesting because it does seem like FTC’s had more immediate results, I guess, than you sometimes see in Washington. [Sen.] Bernie Sanders has piggybacked on what they’re doing and targeted these companies and products in other ways, and gotten some small pricing cost concessions for consumers as well. But it will take a little bit of time for, even if patents get delisted, for generic drugmakers to actually then go through the whole rigamarole of getting cheaper products to market.
Rovner: Yes. This is part of what I call the “30 Years War,” to do something about drug prices. Before we leave drug prices, we’re still fighting in court about the Medicare drug negotiation, right? There, the drug industry continues to lose. Is that where we are?
Karlin-Smith: Correct. They have their fourth negative ruling this week. Basically, in this case, the judge ruled on two main arguments the industry was trying to push forward. One is that the drug negotiation program would constitute a takings violation under the Fifth Amendment. One of the main reasons the judge in this district in New Jersey said no is because they’re saying basically participation in Medicare and this drug price negotiation program are voluntary, the government is not forcibly taking any of your property, you don’t have to participate.
Another big ruling from this judge was that this program does not constitute First Amendment violations. What’s happening here is a regulation of conduct, not speech. One of the more amusing things in the decision to me, that I enjoyed, is the industry has argued that they’re basically being forced under this program to say, “Oh, this is … when CMS [Centers for Medicare & Medicaid Services]” … and then work out a price, that the price they work out is the maximum fair price because that’s the technical terminology used in the law, that they’re then somehow making an admission that any other price that they’ve charged has not been fair. The judge basically said, “Well, this is a public relations problem, not a constitutional problem. Nobody is telling you you can’t go out and publicly disagree with CMS about this program and about their prices that you end up having to enter into.”
It’s another blow. They have a lot of different legal arguments they’re trying out in different cases. As I said, they’ve thrown a lot of spaghetti at the wall. So far, other arguments have failed. Some of the cases are stalled on more technicalities, like the districts they’ve filed in. There was another case that was heard, an appeal was heard yesterday, in PhRMA, the main trade group’s case, where they’re trying to push on because of that. There’s going to be a lot of more action, but so far, looks good for the government.
Pradhan: When this was first rolling out, including when CMS announced the initial 10 drugs that would first be on the list, lawyers that I talked with at the time said that the arguments that the industry was making, it was a reach, to be diplomatic about it. I don’t think anyone really thought that they would be successful and it seems like that is, at least to date, that’s how it’s playing out.
Rovner: I’ll repeat, it’s a good time to be a lawyer for the drug industry, at least you’re very busy.
All right, well, finally this week, we spend so much time talking about how big health care is getting, Walmart this week announced that it’s basically getting out of the primary care business. It’s closing down its two dozen clinics and ending its telehealth programs. This feels like another case of that, “Wow, it looked so easy to make money in health care.” Until you discover that it’s not.
Pradhan: Right. I think making money in primary care, certainly that’s not where the people say, “Oh, that’s a real big cash cow, let’s go in there.” It’s other parts of the health care industry.
Karlin-Smith: One thing that struck me about a quote in a CNN article from Walmart was how they were focusing on they wanted to do this, but they found it wasn’t a sustainable business model. To me, that then just brings up the question of “Should health care be a business?” and the problems. There’s a difference between being able to operate primary care and make enough money to pay your doctors and cover all your costs, and a big company like Walmart that wants to be able to show big returns for their investors and so forth. There’s also that distinction that something that’s not attractive for a business model like that can still be viable in the U.S.
Rovner: This reminds me a lot of ways of the ill-fated Haven Healthcare, which was when Amazon and JPMorgan Chase and Berkshire Hathaway all thought they could get together because they were big, smart companies, could solve health care. They hired Atul Gawande, he was one of the biggest brains in health care, and it didn’t work out. We shall continue.
Anyway, that is the news for this week. Now it’s time for our extra-credit segment. That’s when we each recommend a story we read this week we think you should read, too. As always, don’t worry if you miss it. We will post the links on the podcast page at kffhealthnews.org and in our show notes on your phone or other mobile device.
Rachana, why don’t you go first this week?
Pradhan: This story that I’m going to suggest, [“Millions of American Kids Are Caregivers Now: ‘The Hardest Part Is That I’m Only 17.”] it’s in The Wall Street Journal, depressing like most health care things are. It’s about how millions of children, I think it’s over 5 million children under the age of 18, are providing care to siblings, grandparents, and parents with chronic medical needs, and how they are becoming caregivers at such young ages. In part, because it is so hard to find and afford in-home care for people. That is my extra credit.
Rovner: Right, good story. Sarah?
Karlin-Smith: I looked at a piece in The Atlantic by Katherine J. Wu, “America’s Infectious-Disease Barometer Is Off.” It’s focused on our initial response in this country to bird flu, and maybe where the focus should and shouldn’t be. It has some interesting points about repeat mistakes we seem to be making, in terms of inadequate testing, inadequate focus on the most vulnerable workers, and what we need to do to protect them in this crisis right now.
Rovner: Alice?
Ollstein: I chose [“Dozens of Deaths Reveal Risks of Injecting Sedatives Into People Restrained by Police“] an AP investigation, collaborating with Frontline, about the use of sedatives when police are arresting someone. This is supposed to be a way to safely restrain someone who’s combative, or maybe they’re on drugs, or maybe they’re having a mental health episode, and this is supposed to be a nonlethal way to detain someone. It has led to a lot of deaths, nearly 100 over the past several years. These drugs can make someone’s heart stop. The reporting shows it’s not totally clear if just the drugs themselves are what is killing people, or if it’s in combination with other drugs they might be on, or it’s because they’re being held down in a way by the cops that prevent them from breathing properly, or what. But this is a lot of deaths of people who have received these injections and is leading to discussions of whether this is a best practice. Pretty depressing stuff, but important.
Rovner: Yeah. It was something that was supposed to help and has not so much in many cases. My story this week is from ProPublica. It’s called “A Doctor at Cigna Said Her Bosses Pressured Her To Review Patients’ Cases Too Quickly. Cigna Threatened To Fire Her.” It’s by Patrick Rucker and David Armstrong. It’s about exactly what the headline says. A doctor who spent too much time reviewing potential insurance denials because she wanted to be sure the cases were being decided correctly. It’s obviously not the first story of this kind, but I chose it because it so reminded me of a story that I did in 2007, which was about a physician who worked for a managed-care company, it was Humana in that case, who was pushed to deny care and first testified to Congress about it in 1996. I honestly can’t believe that, 28 years later, we are still arguing about pretty much the exact same types of practices at insurance companies. At some point you would think we would figure out how to solve these things, but apparently not yet.
OK, that is our show. As always, if you enjoy the podcast, you can subscribe wherever you get your podcasts. We’d appreciate it if you left us a review; that helps other people find us, too. Special thanks as always to our technical guru, Francis Ying, and our editor, Emmarie Huetteman. As always, you can email us your comments or questions. We’re at whatthehealth@kff.org, or you can still find me at X @jrovner.
Rachana, where are you hanging these days?
Pradhan: I am also on X, @rachanadpradhan.
Rovner: Sarah?
Karlin-Smith: I’m at @SarahKarlin or @sarahkarlin-smith on Bluesky.
Rovner: Alice?
Ollstein: @AliceOllstein on X, and @alicemiranda on Bluesky.
Rovner: We will be back in your feed next week. Until then, be healthy.
Credits
Francis Ying
Audio producer
Emmarie Huetteman
Editor
To hear all our podcasts, click here.
And subscribe to KFF Health News’ “What the Health?” on Spotify, Apple Podcasts, Pocket Casts, or wherever you listen to podcasts.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
USE OUR CONTENT
This story can be republished for free (details).
1 year 3 months ago
Courts, Multimedia, Pharmaceuticals, States, Abortion, Arizona, Biden Administration, FDA, Florida, KFF Health News' 'What The Health?', Medical Devices, Podcasts, Prescription Drugs, Tobacco, Trump Administration, Women's Health
STAT+: What do CEOs owe the world?
Want to stay on top of the science and politics driving biotech today? Sign up to get our biotech newsletter in your inbox.
Hello! Today, we discuss how the FDA is holding firm in how it handles non-compliant trial sponsors and investigators, how PhRMA is rebuilding its ranks, and offer up a fabulous podcast.
Want to stay on top of the science and politics driving biotech today? Sign up to get our biotech newsletter in your inbox.
Hello! Today, we discuss how the FDA is holding firm in how it handles non-compliant trial sponsors and investigators, how PhRMA is rebuilding its ranks, and offer up a fabulous podcast.
The need-to-know this morning
- Advisers to the European Medicines Agency recommended the approval of Qalsody, a treatment for ALS from Biogen.
What do CEOs owe the world?
Did Humira break the system? And can you CRISPR a fetus? We cover all that and more this week on “The Readout LOUD,” STAT’s biotech podcast.
1 year 5 months ago
Biotech, Business, Health, Pharma, The Readout, Biotech, biotechnology, drug development, drug prices, drug pricing, FDA, Pharmaceuticals, policy, Public Health
Mysterious Morel Mushrooms at Center of Food Poisoning Outbreak
A food poisoning outbreak that killed two people and sickened 51, stemming from a Montana restaurant, has highlighted just how little is known about morel mushrooms and the risks in preparing the popular and expensive delicacy.
The FDA conducted an investigation into morel mushrooms after the severe illness outbreak linked to Dave’s Sushi in Bozeman in late March and April. The investigation found that undercooked or raw morels were the likely culprit, and it led the agency to issue its first guidelines on preparing morels.
“The toxins in morel mushrooms that may cause illness are not fully understood; however, using proper preparation procedures, such as cooking, can help to reduce toxin levels,” according to the FDA guidance.
Even then, a risk remains, according to the FDA: “Properly preparing and cooking morel mushrooms can reduce risk of illness, however there is no guarantee of safety even if cooking steps are taken prior to consumption.”
Jon Ebelt, spokesperson for Montana’s health department, said there is limited public health information or medical literature on morels. And samples of the morels taken from Dave’s Sushi detected no specific toxin, pathogen, pesticide, or volatile or nonvolatile organic compound in the mushrooms.
Aaron Parker, the owner of Dave’s Sushi, said morels are a “boutique item.” In season, generally during the spring and fall, morels can cost him $40 per pound, while morels purchased out of season are close to $80 per pound, he said.
Many highly regarded recipe books describe sauteing morels to preserve the sought-after, earthy flavor. At Dave’s, a marinade, sometimes boiling, was poured over the raw mushrooms before they were served, Parker said. After his own investigation, Parker said he found boiling them between 10 and 30 minutes is the safest way to prepare morel mushrooms.
Parker said he reached out to chefs across the country and found that many, like him, were surprised to learn about the toxicity of morels.
“They had no idea that morel mushrooms had this sort of inherent risk factor regardless of preparation,” Parker said.
According to the FDA’s Food Code, the vast majority of the more than 5,000 fleshy mushroom species that grow naturally in North America have not been tested for toxicity. Of those that have, 15 species are deadly, 60 are toxic whether raw or cooked — including “false” morels, which look like spongy edible morels — and at least 40 are poisonous if eaten raw, but safer when cooked.
The North American Mycological Association, a national nonprofit whose members are mushroom experts, recorded 1,641 cases of mushroom poisonings and 17 deaths from 1985 to 2006. One hundred and twenty-nine of those poisonings were attributed to morels, but no deaths were reported.
Marian Maxwell, the outreach chairperson for the Puget Sound Mycological Society, based in Seattle, said cooking breaks down the chitin in mushrooms, the same compound found in the exoskeletons of shellfish, and helps destroy toxins. Maxwell said morels may naturally contain a type of hydrazine — a chemical often used in pesticides or rocket fuel that can cause cancer — which can affect people differently. Cooking does boil off the hydrazine, she said, “but some people still have reactions even though it’s cooked and most of that hydrazine is gone.”
Heather Hallen-Adams, chair of the toxicology committee of the North American Mycological Association, said hydrazine has been shown to exist in false morels, but it’s not as “clear-cut” in true morels, which were the mushrooms used at Dave’s Sushi.
Mushroom-caused food poisonings in restaurant settings are rare — the Montana outbreak is believed to be one of the first in the U.S. related to morels — but they have happened infrequently abroad. In 2019, a morel food poisoning outbreak at a Michelin-star-rated restaurant in Spain sickened about 30 customers. One woman who ate the morels died, but her death was determined to be from natural causes. Raw morels were served on a pasta salad in Vancouver, British Columbia, in 2019 and poisoned 77 consumers, though none died.
Before the new guidelines were issued, the FDA’s Food Code guidance to states was only that serving wild mushrooms must be approved by a “regulatory authority.”
The FDA’s Food Code bans the sale of wild-picked mushrooms in a restaurant or other food establishment unless it’s been approved to do so, though cultivated wild mushrooms can be sold if the cultivation operations are overseen by a regulatory agency, as was the case with the morels at Dave’s Sushi. States’ regulations vary, according to a 2021 study by the Georgia Department of Public Health and included in the Association of Food and Drug Officials’ regulatory guidelines. For example, Montana and a half-dozen other states allow restaurants to sell wild mushrooms if they come from a licensed seller, according to the study. Seventeen other states allow the sale of wild mushrooms that have been identified by a state-credentialed expert.
The study found that the varied resources states use to identify safe wild mushrooms — including mycological associations, academics, and the food service industry — may suggest a need for better communication.
The study recognized a “guidance document” as the “single most important step forward” given the variety in regulations and the demand for wild mushrooms.
Hallen-Adams said raw morels are known to be poisonous by “mushroom people,” but that’s not common knowledge among chefs.
In the Dave’s Sushi case, Hallen-Adams said, it was obvious that safety information didn’t get to the people who needed it. “And this could be something that could be addressed by labeling,” she said.
There hasn’t been much emphasis placed on making sure consumers know how to properly prepare the mushrooms, Hallen-Adams said, “and that’s something we need to start doing.”
Hallen-Adams, who trains people in Nebraska on mushroom identification, said the North American Mycological Association planned to update its website and include more prominent information about the need to cook mushrooms, with a specific mention of morels.
Montana’s health department intends to publish guidelines on morel safety in the spring, when morel season is approaching.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
USE OUR CONTENT
This story can be republished for free (details).
1 year 7 months ago
Public Health, Rural Health, States, FDA, Food Safety, Montana
The biotech news you missed from the weekend
Want to stay on top of the science and politics driving biotech today? Sign up to get our biotech newsletter in your inbox.
Hello from ASH! Writing this Readout from a press room at the annual hematology confab here in San Diego. Today’s edition is chockfull of Vertex content, plus some extras from ASH and elsewhere.
Want to stay on top of the science and politics driving biotech today? Sign up to get our biotech newsletter in your inbox.
Hello from ASH! Writing this Readout from a press room at the annual hematology confab here in San Diego. Today’s edition is chockfull of Vertex content, plus some extras from ASH and elsewhere.
1 year 7 months ago
Biotech, Business, Health, Pharma, Politics, The Readout, biotechnology, Cancer, drug development, drug pricing, FDA, finance, genetics, Pharmaceuticals, Research
KFF Health News' 'What the Health?': Democrats See Opportunity in GOP Threats to Repeal Health Law
The Host
Julie Rovner
KFF Health News
Julie Rovner is chief Washington correspondent and host of KFF Health News’ weekly health policy news podcast, “What the Health?” A noted expert on health policy issues, Julie is the author of the critically praised reference book “Health Care Politics and Policy A to Z,” now in its third edition.
With other GOP presidential candidates following Donald Trump’s lead in calling for an end to the Affordable Care Act, Democrats are jumping on an issue they think will favor them in the 2024 elections. The Biden administration almost immediately rolled out a controversial proposal that could dramatically decrease the price of drugs developed with federally funded research dollars. The drug industry and the business community at large are vehemently opposed to the proposal, but it is likely to be popular with voters.
Meanwhile, the Supreme Court hears arguments in a case to decide whether the Sackler family should be able to shield billions of dollars taken from its bankrupt drug company, Purdue Pharma, from further lawsuits regarding the company’s highly addictive drug OxyContin.
This week’s panelists are Julie Rovner of KFF Health News, Anna Edney of Bloomberg News, Alice Miranda Ollstein of Politico, and Rachana Pradhan of KFF Health News.
Panelists
Anna Edney
Bloomberg
Alice Miranda Ollstein
Politico
Rachana Pradhan
KFF Health News
Among the takeaways from this week’s episode:
- The ACA may end up back on the proverbial chopping block if Trump is reelected. But as many in both parties know, it is unlikely to be a winning political strategy for Republicans. ACA enrollment numbers are high, as is the law’s popularity, and years after a failed effort during Trump’s presidency, Republicans still have not unified around a proposal to replace it.
- Democrats are eager to capitalize on the revival of “repeal and replace.” This week, the Biden administration announced plans to exercise so-called “march-in rights,” which it argues allow the government to seize certain patent-protected drugs whose prices have gotten too high and open them to price competition. The plan, once largely embraced by progressives, could give President Joe Biden another opportunity to claim his administration has proven more effective than Trump’s heading into the 2024 election.
- The Senate voted to approve more than 400 military promotions this week, effectively ending the 10-month blockade by Republican Sen. Tommy Tuberville of Alabama over a Pentagon policy that helps service members travel to obtain abortions. At the state level, the Texas courts are considering cases over its exceptions to the state’s abortion ban, while in Ohio, a woman who miscarried after being sent home from the hospital is facing criminal charges.
- Meanwhile, the Supreme Court soon could rule on whether EMTALA, or the Emergency Medical Treatment and Active Labor Act, requires doctors to perform abortions in emergencies. And justices are also considering whether to allow a settlement deal to move forward that does not hold the Sacker family accountable for the harm caused by opioids.
- “This Week in Medical Misinformation” highlights a lawsuit filed by Texas Attorney General Ken Paxton accusing Pfizer of failing to end the covid-19 pandemic with its vaccine.
Also this week, Rovner interviews Dan Weissmann, host of KFF Health News’ sister podcast, “An Arm and a Leg,” about his investigation into hospitals suing their patients for unpaid medical bills.
Plus, for “extra credit,” the panelists suggest health policy stories they read this week that they think you should read, too:
Julie Rovner: The Wisconsin State Journal’s “Dane, Milwaukee Counties Stop Making Unwed Fathers Pay for Medicaid Birth Costs,” by David Wahlberg.
Anna Edney: Bloomberg News’ “Tallying the Best Stats on US Gun Violence Is Trauma of Its Own,” by Madison Muller.
Alice Miranda Ollstein: Stat’s “New Abortion Restrictions Pose a Serious Threat to Fetal Surgery,” by Francois I. Luks, Tippi Mackenzie, and Thomas F. Tracy Jr.
Rachana Pradhan: KFF Health News’ “Patients Expected Profemur Artificial Hips to Last. Then They Snapped in Half,” by Brett Kelman and Anna Werner, CBS News.
Also mentioned in this week’s episode:
- Bloomberg News’ “The Pentagon Wants to Root Out Shoddy Drugs. The FDA Is in Its Way,” by Anna Edney and Riley Griffin.
- Ars Technica’s “Texas Sues Pfizer With COVID Anti-Fax Argument That Is Pure Stupid,” by Beth Mole.
Click to open the transcript
Transcript: Democrats See Opportunity in GOP Threats to Repeal Health Law
KFF Health News’ ‘What the Health?’Episode Title: Democrats See Opportunity in GOP Threats to Repeal Health LawEpisode Number: 325Published: Dec. 7, 2023
[Editor’s note: This transcript was generated using both transcription software and a human’s light touch. It has been edited for style and clarity.]
Julie Rovner: Hello, and welcome back to “What the Health?” I’m Julie Rovner, chief Washington correspondent for KFF Health News, and I’m joined by some of the best and smartest reporters in Washington. We’re taping this week on Thursday, Dec. 7, at 10 a.m. As always, news happens fast, and things might’ve changed by the time you hear this. So here we go. Today, we are joined via video conference by Alice Miranda Ollstein of Politico.
Alice Miranda Ollstein: Good morning.
Rovner: Anna Edney of Bloomberg News.
Anna Edney: Hello.
Rovner: And my KFF Health News colleague Rachana Pradhan.
Rachana Pradhan: Good morning, Julie.
Rovner: Later in this episode we’ll have my interview with Dan Weissmann, host of our sister podcast, “An Arm and a Leg.” Dan’s been working on a very cool two-part episode about hospitals suing their patients that he will explain. But first, this week’s news. So now that former President [Donald] Trump has raised the possibility of revisiting a repeal of the Affordable Care Act, all of the other Republican presidential wannabes are adding their two cents.
Florida Gov. Ron DeSantis says that rather than repeal and replace the health law, he would “repeal and supersede,” whatever that means. Nikki Haley has been talking up her anti-ACA bona fides in New Hampshire, and the leading Republican candidate for Senate in Montana is calling for a return to full health care privatization, which would mean getting rid of not only the Affordable Care Act, but also Medicare and Medicaid.
But the Affordable Care Act is more popular than ever, at least judging from this year’s still very brisk open enrollment signups. Alice, you wrote an entire story about how the ACA of 2023 is not the ACA of 2017, the last time Republicans took a serious run at it. How much harder would it be to repeal now?
Ollstein: It would be a lot harder. So, not only have a bunch of red and purple states expanded Medicaid since Republicans took their last swing at the law — meaning that a bunch more constituents in those states are getting coverage they weren’t getting before and might be upset if it was threatened by a repeal — but also just non-Medicaid enrollment is up as well, fueled in large part by all the new subsidies that were implemented over the last few years. And that’s true even in states that resisted expansions.
DeSantis’ Florida, for instance, has the highest exchange enrollment in the country. There’s just a lot more people with a lot more invested in maintaining the program. You have that higher enrollment, you have the higher popularity, and we still haven’t seen a real replacement or “supersede” plan, or whatever they want to call it. And folks I talk to on Capitol Hill, Republican lawmakers, even those that were pretty involved last time, do not think such a plan is coming.
Rovner: It did get asked about at the “last” Republican presidential primary debate last night, and there was an awful lot of hemming and hawing about greedy drug companies and greedy insurance companies, and I heard exactly nothing about any kind of plan. Has anybody else seen any sign of something that Republicans would actually do if they got rid of the Affordable Care Act?
Pradhan: No. There was a time, immediately after the ACA’s passage, that health care was a winning political issue for Republicans, right? It was multiple election cycles that they capitalized on Obamacare and used it to regain House majorities, Senate majorities, and the presidency eventually. But that has not been true for multiple years now. And I think they know that. I think establishment Republicans know that health care is not a winning issue for the party, which is why Democrats are so eager to capitalize on this reopening of ACA repeal, if you will.
Rovner: What a perfect segue, because I was going to say the Biden administration is wasting no time jumping back into health care with both feet, trying to capitalize on what it sees as a gigantic Republican misstep. Just this morning, they are rolling out new proposals aimed at further lowering prescription drug prices, and to highlight the fact that they’ve actually gotten somewhere in some lowering of prescription drug prices.
Now they would like to make it easier to use government “march-in rights,” which would let the government basically tell prescription drug companies, “You’re going to lower your price because we’re going to let other people compete against you, despite your patent.” They’re also doing, and I will use their words, a cross-government public inquiry into corporate greed in health care. Now, some of these things are super controversial. I mean, the march-in rights even before this was unveiled, we saw the drug industry complaining against. But they could also have a real impact if they did some of this, right? Anna, you’ve watched the drug price issue.
Edney: Yeah, I think they definitely could have an impact. This is one of those situations with the march-in rights where we don’t have any clue on where or how exactly, because we haven’t been told that this drug or this class of drugs are kind of what we’re aiming at at this point. It sounds like maybe there’s a little bit more of the plan to be baked, but I am sure there are a lot of progressives, particularly, who had pushed for this that, over the years, who are very excited to even see it mentioned and moving in some sort of way, which hasn’t really happened before.
And, clearly, the Biden administration wants to, like you said, capitalize on health care being part of the campaign. And they’ve done a lot on drug prices, at least a lot in the sense of what can be done. There’s negotiation in Medicare now for some drugs. They kept insulin for Medicare as well. So this is just another step they can say, “We’re doing something else,” and we’d have to see down the line exactly where they’d even plan to use it.
And, of course, as pharma always does, they said that this will hurt innovation and we won’t get any drugs. Not that things have been in place that long, but, clearly, we haven’t seen that so far.
Rovner: Yes, that is always their excuse. I feel like this is one of those times where it doesn’t even matter if any of these things get done, they’re putting them out there just to keep the debate going. This is obviously ground that the Biden campaign would love to campaign on — rather than talking about the economy that makes people mostly unhappy. I assume we’ll be seeing more of this.
Edney: Yeah. Your food prices and other things are very high right now. But if they can talk about getting drug prices lower, that’s a totally different thing that they can point to.
Ollstein: And it’s an easy way to draw the contrast. For people who might be apathetic and think, “Oh, it doesn’t matter who wins the presidential election,” this is an area where the Biden administration can credibly claim, based on what Trump recently said, “This is what’s at stake. This is the difference between my opponent and me. The health care of millions is on the line,” which has been a winning message in past elections.
And what’s been really striking to me is that even talking to a bunch of conservatives now, even though they don’t like the Affordable Care Act, they even are starting to argue that full-scale repeal and replace — now that it’s the status quo — that’s not even a conservative proposal.
They’re saying that it’s more conservative to propose smaller changes that chip around the edges and create some alternatives, but mainly leave it in place, which I think is really interesting, because for so long the litmus test was: Are you for full repeal, root and branch? And we’re just not really hearing that much anymore — except from Trump!
Rovner: The difficulty from the beginning is that the basis of the ACA was a Republican proposal. I mean, they were defanged from the start. It’s been very hard for them to come up with a replacement. What it already is is what Mitt Romney did in Massachusetts. Well, let us turn to the other big issue that Democrats hope will be this coming election year, and that’s abortion, where there was lots of news this week.
We will start with the fact that the 10-month blockade of military promotions by Alabama Republican Sen. Tommy Tuberville is over. Well, mostly over. On Tuesday, the Senate approved by voice vote more than 400 promotions that Tuberville had held up, with only a few four-star nominees still in question. Tuberville’s protests had angered not just Senate Democrats and the Biden administration, who said it was threatening national security, but increasingly his own Senate Republican colleagues.
Tuberville said he was holding up the nominations to protest the Biden administration’s policy of allowing active-duty military members and their dependents to travel out of state for an abortion if they’re stationed where it’s illegal, like in, you know, Alabama. So Alice, what did Tuberville get in exchange for dropping his 10-month blockade?
Ollstein: So, not much. I mean, his aim was to force the Biden administration to change the policy, and that didn’t happen — the policy supporting folks in the military traveling if they’re based in a state where abortion is banned and they need an abortion, supporting the travel to another state, still not paying for the abortion itself, which is still banned. And so that was the policy Tuberville was trying to get overturned, and he did not get that. So he’s claiming that what he got was drawing attention to it, basically. So we’ll see if he tries to use this little bit of remaining leverage to do anything. It does not seem like much was accomplished through this means, although there is a lot of anxiety that this sets a precedent for the future, not just on abortion issues, but, really, could inspire any senator to try to do this and hold a bunch of nominees hostage for whatever policy purpose they want.
Rovner: I know. I mean, senators traditionally sit on nominees for Cabinet posts. And the FDA and the CMS [Centers for Medicare & Medicaid Services] didn’t have a director for, like, three administrations because members were angry at the administration for something about Medicare and Medicaid. But I had never seen anybody hold up military promotions before. This was definitely something new. Rachana, you were going to add something?
Pradhan: Oh, I mean, I was just thinking on Alabama specifically. I mean, I don’t claim to know, even though there was rising anger in Sen. Tuberville’s own party about this move. I mean, I’m not saying I know that this is a factor or not, but in Alabama, regardless of what he tried to do, I think that the attorney general in Alabama has made it clear that he might try to prosecute organizations that help people travel out of state to get abortions.
And so, it’s not like this is only the last word when you’re even talking about military officers or people in the military. Even in his home state, you might see some greater activity on that anyway, which might make it easier for him to honestly, in a way, give it up because it’s not the only way that you could presumably prosecute organizations or people who tried to help others go out of state to access abortion.
Rovner: Yeah, it’s important to say that while he irritated a lot of people in Washington, he probably had a lot of support from people back home in Alabama, which he kept pointing out.
Ollstein: Right. And I saw national anti-abortion groups really cheering him on and urging their members to send him thank-you letters and such. And so definitely not just in his home state. There are conservatives who were backing this.
Rovner: Well, moving on to Texas, because there is always abortion news out of Texas, we have talked quite a bit about the lawsuit filed by women who experienced pregnancy complications and couldn’t get abortions. Well, now we have a separate emergency lawsuit from a woman named Kate Cox, who is currently seeking an abortion because of the threat to her health and life.
Both of these lawsuits aren’t trying to strike the Texas ban, just to clarify when a doctor can perform a medically needed abortion without possibly facing jail time or loss of their medical license. Alice, I know the hearing for Kate Cox is happening even now as we are taping. What’s the status of the other case? We’re waiting to hear from the Texas Supreme Court. Is that where it is?
Ollstein: Yeah. So oral arguments were the other day and a bunch of new plaintiffs have joined the lawsuit. So it’s expanded to a few dozen people now, mostly patients, but some doctors as well who are directly impacted by the law. There was some interesting back and forth in the oral arguments over standing.
And one of the things the state was hammering was that they don’t have standing to sue because they aren’t in this situation that this other woman is in today, where they’re actively pregnant, actively in crisis, and being actively prevented from accessing the health care that they need that their doctor recommends, which in some circumstances is an abortion. And so I think this is an interesting test of the state’s argument on that front.
Rovner: Also, the idea, I mean, that a woman who literally is in the throes of this crisis would step forward and have her name in public and it’s going to court in an emergency hearing today.
Ollstein: Right, as opposed to the other women who were harmed previously. By the time they are seeking relief in court, their pregnancy is already over and the damage has already been done, but they’re saying it’s a threat of a future pregnancy. It’s impacting their willingness to become pregnant again, knowing what could happen, what already happened. But the state was saying like, “Oh, but because you’re not actively in the moment, you shouldn’t have the right to sue.” And so now we’ll see what they say when someone is really in the throes of it.
Rovner: In the moment. Well, another troubling story this week comes from Warren, Ohio, where a woman who experienced a miscarriage is being charged with “abuse of a corpse” because she was sent home from the hospital after her water broke early and miscarried into her toilet, which is gross, but that’s how most miscarriages happen.
The medical examiner has since determined that the fetus was, in fact, born dead and was too premature to survive anyway. Yet the case seems to be going forward. Is this what we can expect to see in places like Ohio where abortion remains legal, but prosecutors want to find other ways to punish women?
Ollstein: I mean, I also think it’s an important reminder that people were criminalized for pregnancy loss while Roe [v. Wade] was still in place. I mean, it was rare, but it did happen. And there are groups tracking it. And so I think that it’s not a huge surprise that it could happen even more now, in this post-Roe era, even in states like Ohio that just voted overwhelmingly to maintain access to abortion.
Pradhan: Julie, do we know what hospital? Because when I was looking at the story, do we know what kind of hospital it was that sent this person away?
Rovner: No. The information is still pretty sketchy about this case, although we do know the prosecutor is sending it to a grand jury. We know that much. I mean, the case is going forward. And we do know that her water broke early and that she did visit, I believe, it was two hospitals, although I have not seen them named. I mean, there’s clearly more information to come about this case.
But yeah, Alice is right. I mean, I wrote about a case in Indiana that was in 2012 or 2013, it was a long time ago, about a woman who tried to kill herself and ended up only killing her fetus and ended up in jail for a year. I mean, was eventually released, but … it’s unusual but not unprecedented for women to be prosecuted, basically, for pregnancy loss.
Ollstein: Yeah, especially people who are struggling with substance abuse. That’s been a major area where that’s happened.
Pradhan: I would personally be very interested in knowing the hospitals that are a part of this and whether they are religiously affiliated, because there’s a standard of care in medicine for what happens if you have your water break before the fetus is viable and what’s supposed to happen versus what can happen.
Rovner: There was a case in Michigan a few years ago where it was a Catholic hospital. The woman, her water broke early. She was in a Catholic hospital, and they also sent her home. I’m trying to remember where she finally got care. But yeah, that has been an issue also over the years. Well, meanwhile, back here in Washington, the Supreme Court is likely to tell us shortly, I believe, whether the 1986 Emergency Medical Treatment and Active Labor Act, known as EMTALA, requires doctors to perform abortions in life-threatening situations, as the Biden administration maintains.
Alice, this case is on what’s known as the “shadow docket” of the Supreme Court, meaning it has not been fully briefed and argued. It’s only asking if the court will overturn a lower court’s ruling that the federal law trumps the state’s ban. When are we expecting to hear something?
Ollstein: It could be after justices meet on Friday. Really, it could be whenever after that. As we’ve seen in the last few years, the shadow docket can be very unpredictable, and we could just get, at very odd times, major decisions that impact the whole country or just one state. And so, yes, I mean, this issue of abortion care and emergency circumstances is playing out in court in a couple different states, and the federal government is getting involved in some of those states.
And so I think this could be a big test. Unlike a lot of lawsuits going on right now, this is not seeking to strike down the state’s abortion ban entirely. It’s just trying to expand and clarify that people who are in the middle of a medical emergency shouldn’t be subject to the ban.
Rovner: It’s similar to what they’re fighting about in Texas, actually.
Ollstein: Yeah, exactly. And this is still playing out at the 9th Circuit, but they’re trying to leapfrog that and get the Supreme Court to weigh in the meantime.
Rovner: Yeah, and we shall see. All right, well, while we’re on the subject of “This Week in Court,” let us move on to the case that was argued in public at the Supreme Court this week about whether the Sackler family can keep much of its wealth while declaring bankruptcy for its drug company, Purdue Pharma, that’s been found liable for exacerbating, if not causing much of the nation’s opioid epidemic.
The case involves basically two bad choices: Let the Sacklers manipulate the federal bankruptcy code to shield billions of dollars from future lawsuits or further delay justice for millions of people injured by the company’s behavior. And the justices themselves seem pretty divided over which way to go. Anna, what’s at stake here? This is a lot, isn’t it?
Edney: Yeah. I mean, it’s interesting how it doesn’t exactly break down on ideological lines. The justices were — I don’t want to say all over the place, because that sounds disrespectful — but they had concerns on many different levels. And one is that the victims and their lawyers negotiated the settlement because for them it was the best way they felt that they could get compensation, and they didn’t feel that they could get it without letting the Sacklers off the hook, that the Sacklers basically would not sign the settlement agreement, and they were willing to go that route.
And the government is worried about using that and letting the Sacklers off the hook in this way and using this bankruptcy deal to be able to shield a lot of their money that they took out of the company, essentially, and have in their personal wealth now. And so that’s something that a lot of companies are, not a lot, but companies are looking to hope to use the sign of bankruptcy protection when it comes to big class-action lawsuits and harm to consumers.
And so I think that what the worry is is that that then becomes the precedent, that the ones at the very top will always get off because it’s easier to negotiate the settlement that way.
Rovner: We’ll obviously have to wait until — as this goes a few months — to see the decisions in this case, but it’s going to be interesting. I think everybody, including the justices, are unhappy with the set of facts here, but that’s why it was in front of the Supreme Court. So our final entry in “This Week in Court” is a twofer. It is also “This Week in Health Misinformation.”
Texas Attorney General Ken Paxton has filed suit against Pfizer for allegedly violating Texas’ Deceptive Trade Practices Act because its covid vaccine did not, in fact, end the covid epidemic. Quoting from the attorney general’s press release, “We are pursuing justice for the people of Texas, many of whom were coerced by tyrannical vaccine mandates to take a defective product sold by lies.” It’s hard to even know where to start with this, except that, I guess, anyone can sue anyone for anything in Texas, right?
Edney: Yeah, that’s a very good point. The entire concept of it feels so weird. I mean, a vaccine doesn’t cure anything, right? That’s not the point of a vaccine. It’s not a drug. It is a vaccine that is supposed to prevent you from getting something, and not everybody took it. So that feels like the end of the story, but, clearly, the attorney general would prefer attention, I think, on this, and to continue to sow doubt in vaccines and the government and the Food and Drug Administration seems to be maybe more of the point here.
Rovner: I noticed he’s only suing for, I think, it’s $10 million, which is frankly not a ton of money to a company as big as Pfizer. So one would assume that he’s doing this more for the publicity than for the actual possibility of getting something.
Pradhan: Yeah, I think Pfizer’s CEO’s annual salary is more than the damages that are being sought in this case. So it’s really not very much money at all. I mean, more broadly speaking, I mean, Texas, Florida, I think you see especially post-public-health-emergency-covid times, the medical freedom movement has really taken root in a lot of these places.
And I think that it just seems like this is adding onto that, where doctors say they should be able to give ivermectin to covid patients and it helped them and not be at risk of losing their license. And that’s really kind of an anti-vaccine sentiment. Obviously, it’s very alive and well.
Rovner: We are post-belief-in-scientific-expertise.
Edney: Well, I was going to say, I appreciated The Texas Tribune’s story on this because they called out every time in this lawsuit that he was twisting the truth or just completely not telling the truth at all in the sense that he said that more people who took the vaccine died, and that’s clearly not the case. And so I appreciated that they were trying to call him out every time that he said something that wasn’t true, but was just completely willing to put that out in the public sphere as if it was.
Rovner: There was also a great story on Ars Technica, which is a scientific website, about how the lawsuit completely misrepresents the use of statistics, just got it completely backwards. We’ll post a link to that one too. Well, while we are talking about drug companies, let’s talk about some drugs that really may not be what companies say they are.
Anna, you have a new story up this week about the Pentagon’s effort to ensure that generic drugs are actually copies of the drugs they’re supposed to be. That effort’s running into a roadblock. Tell us a little about that.
Edney: Yeah, thanks for letting me talk about this one. It’s “The Pentagon Wants to Root Out Shoddy Drugs. The FDA Is in Its Way.” So the FDA is the roadblock to trying to figure out whether the drugs, particularly that the military and their family members are taking, work well and don’t have side effects that could be extremely harmful. So what’s going on here is that the Defense Department and others, the White House even, has grown skeptical of the FDA’s ability to police generic drugs, largely that are made overseas.
We did some analysis and we found that it was actually 2019 was the first time that generic drug-making facilities in India surpassed the number of those in the U.S. So we are making more, not just active ingredients, but finished products over in India. And the FDA just doesn’t have a good line into India. They don’t do many unannounced inspections. They usually have to tell the company they’re coming weeks in advance. And what we found is when the Defense Department started looking into this, they partnered with a lab to test some of these drugs.
They got some early results. Those results were concerning, as far as the drug might not work, and also could cause kidney failure, seizures. And even despite this, they’ve been facing the FDA around every corner trying to stop them and trying to get them not to test drugs. They say it’s a waste of money, when, in fact, Kaiser Permanente has been doing this for its 12.7 million members for several years.
And it just seems like something is going on at the FDA and that they don’t want people to have any questions about generic drugs. They really just want everyone to accept that they’re always exactly the same, and they even derail the White House effort to try to look into this more as well. But the Pentagon said, “Thank you very much, FDA, but we’re going forward with this.”
Rovner: Yeah. I mean, I could see that the FDA would be concerned about … they’re supposed to be the last word on these things. But, as you point out and as much of your reporting has pointed out over the last couple of years, the FDA has not been able to keep up with really making sure that these drugs are what they say they are.
Edney: One thing I learned that was interesting, and this is that in Europe, actually, there’s a network of 70 labs that do this kind of testing before drugs reach patients and after they reach patients. So it’s not a totally unusual thing. And for some reason, the FDA does not want that to happen.
Rovner: Well, finally on the drug beat this week, CVS announced earlier that it would overhaul its drug pricing to better reflect how much it pays for the drugs, all of which sounds great, but the fact is that how much CVS pays for the drugs doesn’t have all that much effect on how much we end up paying CVS for those same drugs, right? They’re just changing how they get the drugs from the manufacturers, not necessarily how they price it for the customers.
Pradhan: I think my main question would be, what does that mean for a patient’s out-of-pocket cost for prescriptions? I don’t know how much of this has to do with … for CVS as the pharmacy, but we have CVS Caremark, which is a major PBM, and how this affects the pricing models there. And PBMs, of course, have been under scrutiny in Congress. And there’s outside pressure too, right? The story that you highlighted, Julie, talks about Mark Cuban’s affordable-drug effort. And so, yeah, I don’t know. I mean, I think it sounds good until maybe we see some more details, right?
Rovner: I saw one story that said, “This could really help lower drug prices for consumers,” and one that said, “This could actually raise drug prices for consumers.” So I’m assuming that this is another one where we’re going to have to wait and see the details of. All right, well, that is this week’s news.
Now we will play my interview with Dan Weissmann of the “Arm and a Leg” podcast, and then we will come back with our extra credits. I am pleased to welcome back to the podcast Dan Weissmann, host of KFF Health News’s sister podcast, “An Arm and a Leg.” Dan has a cool two-part story on hospitals suing patients for overdue bills that he’s here to tell us about. Dan, welcome back.
Dan Weissmann: Julie, thanks so much for having me.
Rovner: So over the past few years, there have been a lot of stories about hospitals suing former patients, including a big investigation by KFF Health News. But you came at this from kind of a different perspective. Tell us what you were trying to find out.
Weissmann: We were trying to figure out why hospitals file lawsuits in bulk. Investigative reporters like Jay Hancock at KFF [Health News] have documented this practice, and one of the things that they note frequently is how little money hospitals get from these lawsuits. Jay Hancock compared the amount that VCU [Health] was seeking from patients and compared it to that hospital system’s annual surplus, their profit margin, and it looked tiny. And other studies document essentially the same thing. So why do they do it?
And we got a clue from a big report done by National Nurses United in Maryland, which, in addition to documenting how little money hospitals were getting compared to the million-dollar salaries they were paying executives in this case, also noted that a relatively small number of attorneys were filing most of these lawsuits. Just five attorneys filed two-thirds of the 145,000 lawsuits they documented across 10 years, and just one attorney filed more than 40,000 cases. So we were like, huh, maybe that’s a clue. Maybe we found somebody who is getting something out of this. We should find out more.
Rovner: So you keep saying “we” — you had some help working on this. Tell us about your partners.
Weissmann: Oh my God, we were so, so lucky. We work with The Baltimore Banner, which is a new daily news outlet in Baltimore, new nonprofit news outlet in Baltimore, that specializes in data reporting. Their data editor, Ryan Little, pulled untold numbers of cases, hundreds of thousands of cases, from the Maryland courts’ website and analyzed them to an inch of their life and taught us more than I could ever have imagined.
And Scripps News also came in as a partner and one of their data journalists, Rosie Cima, pulled untold numbers of records from the Wisconsin court system and worked to analyze data that we also got from a commercial firm that has a cache of data that has more detail than what we could pull off the website. It was a heroic effort by those folks.
Rovner: So what did you find? Not what you were expecting, right?
Weissmann: No. While Rosie and Ryan were especially gathering all this data and figuring out what to do with it, I was out talking to a lot of people. And what I found out is that in the main, it appears that frequently when these lawsuits happen and when hospitals file lawsuits in general, they’re not being approached by attorneys. They’re working with collection agencies. And most hospitals do work with a collection agency, and it’s essentially like, I put it like, you get a menu, oh, I’m having a hamburger, I’m going to pursue people for bills.
Like, OK, do you want onions? Do you want mustard? Do you want relish? What do you want on it? And in this case, it’s like, how hard do you want us to go after people? Do you want us to hit their credit reports — if you still can do that, because the CFPB [Consumer Financial Protection Bureau] has been making regulations about that — but do you want us to do that? How often can we call them? And do you want us to file lawsuits if we don’t get results? And so that is essentially in consultation with the hospital’s revenue department and the collection agency, and it’s a strategic decision between them.
That was what we found out through talking to people. What Rosie and Ryan turned up, and the data we had from the folks in New York backed up, is that, surprisingly, in the three states that we looked at, there’s just so much less of this activity than we had expected to find. In Maryland, Ryan sent me a series of emails, the first saying, like, “I’m not actually seeing any this year. That’s got to be wrong. They must be hiding them somewhere. I’m going to go investigate.” And a week later he was like, “I think I found them, and then we’ll go run some more numbers.”
And then a week later, after going to the courthouse and looking at everything you could find, he was like, “No, actually Maryland hospitals just do not seem to be suing anybody this year.” And we had expected there to be fewer lawsuits, but zero was a surprise to everybody. In New York, we appear to have found that two of those three law firms handling all those cases are no longer handling medical bill cases. And in Wisconsin, final numbers are still being crunched. Our second part will have all those numbers.
The Banner is coming out with their numbers this week. But Scripps News and us are still crunching numbers in Wisconsin. But what was the biggest shocker was, I can just tell you, there were so many fewer lawsuits than we had expected, and many of the most active plaintiffs had either cut the practice entirely, like filing zero lawsuits or filing hardly any. One of the things that a lot of these reports that look at across a state, like in New York and the Maryland report, note, and that we found in Wisconsin too, is that most hospitals don’t do this.
Noam Levey at KFF [Health News] found that many hospitals have policies that say, “We might file a lawsuit,” and some larger number of hospitals file some lawsuits. But in all these cases where you’re seeing tons of lawsuits filed, the phenomenon of suing people in bulk is actually not business as usual for most hospitals. That is driven by a relatively small group of players. There was a study in North Carolina by the state treasurers, obviously and Duke University, that found 95% of all the lawsuits were filed by just a few institutions.
The New York people found this. We’ve seen it in Wisconsin. So I mean, it’s another very interesting question when you’re looking at why does this happen. It’s like it’s not something that most institutions do. And again, in Wisconsin, we found that most of the players that had been the most active had basically stopped.
Rovner: Do we know why? Is it just all of the attention that we’ve seen to this issue?
Weissmann: Probably the answer is we don’t know why. Our colleagues at The Banner called every hospital in Maryland and were not told very much. We emailed all the hospitals in Wisconsin that we could that we had seen dramatically decrease, and nobody came to the phone. So we don’t really know.
But it does seem like, certainly in Maryland and New York, there were these huge campaigns that got tons of publicity and got laws changed, got laws passed. And there has been attention. The reports that Bobby Peterson put out in Wisconsin got attention locally. Sarah Kliff of The New York Times, who’s been writing about these kinds of lawsuits, has written multiple times about hospitals in Wisconsin. So it seems like a good first guess, but it’s a guess. Yeah.
Rovner: Well, one thing that I was interested that you did turn up, as you pointed out at the top, hospitals don’t get very much money from doing this. You’re basically suing people for money that they don’t have. So you did find other ways that hospitals could get reimbursed. I mean, they are losing a lot of money from people who can’t pay, even people with insurance, who can’t pay their multi-thousand-dollar deductible. So what could they be doing instead?
Weissmann: They could be doing a better job of evaluating people’s ability to pay upfront. The majority of hospitals in the United States are obligated by the Affordable Care Act to have charity care policies, financial assistance policies, in which they spell out, if you make less than a certain amount of money, it’s a multiple of the federal poverty level that they choose, we’ll forgive some or all of your bill. And, frequently, that number is as much as four times the federal poverty level. They might knock 75% off your bill, which is a huge help.
And as a guy that I met noted, using data from KFF, 58% of Americans make less than 75% of the federal poverty level. That is a lot of people. And so if you’re chasing someone for a medical bill, they might very well have been someone you could have extended financial assistance to. This guy, his name is Nick McLaughlin, he worked for 10 years for a medical bill collections agency. Someone in his family had a medical bill they were having a hard time paying, and he figured out that they qualified for charity care, but the application process, he noted, was really cumbersome, and even just figuring out how to apply was a big process. And so he thought, I know that chasing people for money they don’t have isn’t really the best business model and that we’re often chasing people for money they don’t have. What if we encourage hospitals to be more proactive about figuring out if someone should be getting charity care from them in the first place?
Because, as he said, every time you send someone a bill, you’re spending two bucks. And you’re not just sending one bill, you’re sending like three bills and a final notice. That all adds up, and you’re manning a call center. You’re spending money and you’re missing opportunities by not evaluating people. Because while you’re asking about their income, you might find out they’re eligible for Medicaid, and you can get paid by Medicaid rather than chasing them for money they don’t have.
And two, they might update their insurance information from you and you can get money from their insurance. You can extend financial assistance to somebody, as you said, who has a deductible they can’t pay, and they might actually come to you for care that you can unlock money from their insurance if they’re going to come to you because they’re not afraid of the bill.
I should absolutely say, while Nick McLaughlin is selling hospitals on the idea of adopting new software, which is a great idea, they should do that, they should be more proactive, an entity called Dollar For, a nonprofit organization out of the Pacific Northwest that’s been doing work all over the country, has been beating the drum about this and has a tool that anybody can use.
Essentially, go to their website, dollarfor.org, and type in where you were seen and an estimate of your income, and they will tell you, you’re likely to qualify for charity care at this hospital, because they have a database of every hospital’s policy. And if you need help applying, because some of these applications are burdensome, we’ll help you. So this exists, and everybody should know about it and everybody should tell everybody they know about it. I think the work they’re doing is absolutely heroic.
Rovner: Well, Dan Weissmann, thank you so much for joining us. We will post a link to Dan’s story in our show notes and on our podcast page.
Weissmann: Julie, thanks so much for having me.
Rovner: OK, we are back. And it’s time for our extra-credit segment. That’s when we each recommend a story we read this week we think you should read, too. As always, don’t worry if you miss it. We will post the links on the podcast page at kffhealthnews.org and in our show notes on your phone or other mobile device. Anna, why don’t you go first this week?
Edney: Sure. This is from my colleague Madison Muller, “Tallying the Best Stats on US Gun Violence Is Trauma of Its Own.” And I thought she just did such an amazing job with this story, talking to Mark Bryant, who helped start an organization, Gun Violence Archive, which is essentially the only place that is trying to tally every instance of gun violence.
And because of a lot of the restrictions that the NRA has helped get into government regulations and things, some of them which are more recently loosening, but because of those in the past, this is really the only way you could try to look up these statistics. And he’s just given the last decade of his life, with no breaks, trying to do this and his health is failing. And I thought it was just a really poignant look at somebody who has no skin in the game, but just wanted the right information out there.
Rovner: Yeah, obviously this is a big deal. Alice.
Ollstein: I did an op-ed that was published in Stat by a group of fetal medicine specialists who are writing about how their work is being compromised by state abortion bans right now. They were saying these are very risky, high-stakes procedures where they perform operations in utero, latent pregnancy usually, and it’s an attempt to save the pregnancy where there is a big risk. But with all of these, there are risks that it could end the pregnancy, and now they’re afraid of being prosecuted for that.
And they describe a bunch of challenging situations that, even without these bans are challenging, things where there’s twins and something to help one could harm the other twin, and this could all affect the health and life of the parent as well. And so they’re saying that they’re really in this whole new era and have to think about the legal risks, as well as the medical and bioethical ones that they already have to deal with.
Rovner: I’ve reported about this over the years, and I can tell you that these are always really wrenching family decisions about trying to desperately save a pregnancy by doing this extraordinarily difficult and delicate kind of procedure. Rachana.
Pradhan: My extra credit is a story from our colleague Brett Kelman, who worked on this investigation with CBS News. It is about a type of artificial hip known as Profemur that literally were snapping in half in patients’ bodies. I told Brett earlier this week that I was cringing at every line that I read. So if folks want to get a really, frankly, pretty gruesome, awful story about how people around the country have received these artificial hips, and the fact that they broke inside their bodies has really caused a lot of damage.
And, frankly, I know we talked about the FDA, but also this story really sheds light on how the FDA has dropped the ball in not acting with more urgency. And had they done that, many of these injuries likely would’ve been avoided. So, I urge everyone to read it. It’s a great story.
Rovner: It is. I also flinched when I was reading a lot of it. Well, my story is from the Wisconsin State Journal by David Wahlberg, and it’s called “Dane, Milwaukee Counties Stop Making Unwed Fathers Pay for Medicaid Birth Costs.” And while I have been covering Medicaid since the 1980s, and I never knew this even existed, it seems that a handful of states, Wisconsin among them, allows counties to go after the fathers of babies born on Medicaid, which is about half of all births — Medicaid’s about half of all births.
Not surprisingly, making moms choose between disclosing the father to whom she is not married to the state or losing Medicaid for her infant is not a great choice. And there’s lots of research to suggest that it can lead to bad birth outcomes, particularly in African American and Native American communities. I have long known that states can come after the estates of seniors who died after receiving Medicaid-paid nursing home or home care, but this one, at the other end of life, was a new one to me.
Now, I want to know how many other states are still doing this. And when I find out, I’ll report back.
OK, that is our show. As always, if you enjoy the podcast, you can subscribe wherever you get your podcast. We’d appreciate it if you left us a review. That helps other people find us too. Thanks, as always, to our tireless tech guru, Francis Ying, who’s back from vacation. Also, as always, you can email us your comments or questions. We’re at whatthehealth@kff.org, or you can still find me at X, @jrovner, or @julierovner at Bluesky and Threads. Anna?
Edney: @anna_edneyreports on Threads and @annaedney on X.
Rovner: Rachana.
Pradhan: I’m @rachanadpradhan on X.
Rovner: Alice.
Ollstein: I’m @AliceOllstein on X, and @AliceMiranda on Bluesky.
Rovner: We will be back in your feed next week. Until then, be healthy.
Credits
Francis Ying
Audio producer
Emmarie Huetteman
Editor
To hear all our podcasts, click here.
And subscribe to KFF Health News’ “What the Health?” on Spotify, Apple Podcasts, Pocket Casts, or wherever you listen to podcasts.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
USE OUR CONTENT
This story can be republished for free (details).
1 year 7 months ago
Elections, Health Care Costs, Health Care Reform, Health Industry, Medicaid, Medicare, Multimedia, States, Abortion, Biden Administration, FDA, KFF Health News' 'What The Health?', Legislation, Obamacare Plans, Podcasts, Women's Health
KFF Health News' 'What the Health?': Trump Puts Obamacare Repeal Back on Agenda
The Host
Julie Rovner
KFF Health News
Julie Rovner is chief Washington correspondent and host of KFF Health News’ weekly health policy news podcast, “What the Health?” A noted expert on health policy issues, Julie is the author of the critically praised reference book “Health Care Politics and Policy A to Z,” now in its third edition.
Former president and current 2024 Republican front-runner Donald Trump is aiming to put a repeal of the Affordable Care Act back on the political agenda, much to the delight of Democrats, who point to the health law’s growing popularity.
Meanwhile, in Texas, the all-Republican state Supreme Court this week took up a lawsuit filed by more than two dozen women who said their lives were endangered when they experienced pregnancy complications due to the vague wording of the state’s near-total abortion ban.
This week’s panelists are Julie Rovner of KFF Health News, Joanne Kenen of Johns Hopkins University and Politico Magazine, Victoria Knight of Axios, and Sarah Karlin-Smith of the Pink Sheet.
Panelists
Joanne Kenen
Johns Hopkins Bloomberg School of Public Health and Politico
Victoria Knight
Axios
Sarah Karlin-Smith
Pink Sheet
Among the takeaways from this week’s episode:
- The FDA recently approved another promising weight loss drug, offering another option to meet the huge demand for such drugs that promise notable health benefits. But Medicare and private insurers remain wary of paying the tab for these very expensive drugs.
- Speaking of expensive drugs, the courts are weighing in on the use of so-called copay accumulators offered by drug companies and others to reduce the cost of pricey pharmaceuticals for patients. The latest ruling called the federal government’s rules on the subject inconsistent and tied the use of copay accumulators to the availability of cheaper, generic alternatives.
- Congress will revisit government spending in January, but that isn’t soon enough to address the end-of-the-year policy changes for some health programs, such as pending cuts to Medicare payments for doctors.
- “This Week in Medical Misinformation” highlights a guide by the staff of Stat to help lay people decipher whether clinical study results truly represent a “breakthrough” or not.
Also this week, Rovner interviews KFF Health News’ Rachana Pradhan, who reported and wrote the latest “Bill of the Month” feature, about a woman who visited a hospital lab for basic prenatal tests and ended up owing almost $2,400. If you have an outrageous or baffling medical bill you’d like to share with us, you can do that here.
Plus, for “extra credit,” the panelists suggest health policy stories they read this week that they think you should read, too:
Julie Rovner: KFF Health News’ “Medicaid ‘Unwinding’ Makes Other Public Assistance Harder to Get,” by Katheryn Houghton, Rachana Pradhan, and Samantha Liss.
Joanne Kenen: KFF Health News’ “She Once Advised the President on Aging Issues. Now, She’s Battling Serious Disability and Depression,” by Judith Graham.
Victoria Knight: Business Insider’s “Washington’s Secret Weapon Is a Beloved Gen Z Energy Drink With More Caffeine Than God,” by Lauren Vespoli.
Sarah Karlin-Smith: ProPublica’s “Insurance Executives Refused to Pay for the Cancer Treatment That Could Have Saved Him. This Is How They Did It,” by Maya Miller and Robin Fields.
Also mentioned in this week’s episode:
- KFF Health News’ “Progressive and Anti-Abortion? New Group Plays Fast and Loose to Make Points,” by Darius Tahir.
- ProPublica’s “Some Republicans Were Willing to Compromise on Abortion Ban Exceptions. Activists Made Sure They Didn’t,” by Kavitha Surana.
click to open the transcript
Transcript: Trump Puts Obamacare Repeal Back on Agenda
KFF Health News’ ‘What the Health?’Episode Title: Trump Puts Obamacare Repeal Back on AgendaEpisode Number: 324Published: Nov. 30, 2023
[Editor’s note: This transcript was generated using both transcription software and a human’s light touch. It has been edited for style and clarity.]
Julie Rovner: Hello, and welcome back to “What the Health?” I’m Julie Rovner, chief Washington correspondent for KFF Health News, and I’m joined by some of the best and smartest health reporters in Washington. We’re taping this week on Thursday, Nov. 30, at 10 a.m. As always, news happens fast, and things might’ve changed by the time you hear this. So, here we go.
We are joined today via video conference by Sarah Karlin-Smith of the Pink Sheet.
Sarah Karlin-Smith: Hi, Julie.
Rovner: Victoria Knight of Axios News.
Victoria Knight: Hello, everyone.
Rovner: And Joanne Kenen of the Johns Hopkins University and Politico Magazine.
Joanne Kenen: Hi, everybody.
Rovner: Later in this episode we’ll have my interview with my colleague Rachana Pradhan about the latest KFF Health News-NPR “Bill of the Month.” This month’s patient fell into an all-too-common trap of using a lab suggested by her doctor’s office for routine bloodwork without realizing she might be left on the hook for thousands of dollars. But first, this week’s news — and last week’s, too, because we were off.
Because nothing is ever gone for good, the effort to repeal and replace Obamacare is back in the news, and it’s coming primarily from the likely Republican presidential nominee, Donald Trump. Just to remind you, in case you’ve forgotten, Trump, during his presidency, even in the two years that Republicans controlled the House and the Senate, was unable to engineer a repeal of the Affordable Care Act, nor did his administration even manage to unveil an alternative. So what possible reason could he have for thinking that this is going to help him politically now?
Knight: My takeaway is that I think it’s a personal grudge that former President Trump still has, that he failed at this. And I think, when you talk to people, he’s still mad that Sen. John McCain did his famous thumbs-down when the rest of the Republican Party was on board. So I’m not sure that there is much political strategy besides wanting to just make it happen finally, because upset it didn’t happen.
Rovner: Is this part of his revenge tour?
Knight: I mean, I think somewhat. Because if you ask House Freedom Caucus people, they will say, “Yeah, we should repeal it.” But if you ask some more moderate Republican members, they’re like, “We’ve already been through that. We don’t want to do it again.” So I don’t think the Republican Party on the Hill has an appetite to do that, even if Congress goes to Republicans in both chambers.
Kenen: Trump never came up with a health plan and repeal died in the Senate, but remember, it was a struggle to even get anything through the House, and what the House Republicans finally voted for, they didn’t even like. So I don’t know if you call this a revenge tour, but it’s checking a box. But I think it’s important to remember that if you look closely at what Republican policies are, they don’t call it repeal, they don’t say, “We are going to repeal it.” That didn’t go so well for them, and it probably cost them an election.
But they still do have a lot of policy ideas that would water down or de facto repeal many key provisions of Obamacare. So they haven’t tried to go that route, and I’m not sure they would ever try a full-out repeal, but there are lots of other things they could do, some of which would have technical names: community rating and things like that, that voters might not quite understand what they were doing, that could really undermine the protections of Obamacare.
Rovner: Yeah, I mean, I was going to say the Republican Party, in general, this has been the running joke since they started “repeal and replace” in 2010, is that they haven’t had the “replace” part of “repeal and replace” at all. Trump kept saying he was going to have a great plan, it’s coming in two weeks, and, of course, now he’s saying he’s going to have a great plan. We’ve never seen this great plan because the Republicans have never been able to agree on what should come next. Aside from, as Joanne says, tinkering around with the Affordable Care Act.
Kenen: Some of that tinkering would be significant.
Rovner: It could be.
Kenen: I mean there are things that they could tinker that wouldn’t be called repeal, but would actually really make the ACA not work very well.
Rovner: But most of the things that the Republicans wanted to do to the ACA have already been done, like repealing the individual mandate, getting rid of a lot of the industry-specific taxes that they didn’t like.
Kenen: Right. So they ended up getting rid of the spinach and they end up with the stuff that even Republicans, they might not say they like the ACA, but they’re being protected by it. And the individual mandate was the single-most unpopular, contentious part of the law and even a lot of Democrats didn’t like it. And so that target of the animus is gone. So by killing part of it, they also made it harder to do things in the future. They could do damage, though.
Rovner: Yeah. Or they could take on entitlements which, of course, is where the real money is. But we’ll get to that in a minute. Sarah, we have not seen you in a while, so we need to catch up on a bunch of things that are FDA-related. First, a couple of payment items since you were last here. The FDA has, as expected, approved a weight loss version of the diabetes drug Mounjaro that appears to be even more effective than the weight loss version of Ozempic. But insurers are still very reluctant to pay for these drugs, which are not only very expensive, they appear to need to be consumed very long-term, if not forever. Medicare has so far resisted calls to cover the drugs, despite some pressure from members of Congress, but that might be about to change.
Karlin-Smith: I think Medicare is getting a lot of pressure. They’re going to have to probably re-look at it at some point. What I found interesting is recently CMS [the Centers for Medicare and Medicaid Services] regulates other types of health plans as well, and in the ACA space they seem to be pushing for coverage of these obesity drugs. And I think they’re thinking around that. They note that the non-coverage allowance for these ACA plans was based on … they were following what Medicare was doing and there’s some acknowledgment that maybe the non-Medicare population is different from the Medicare population. But I think it’s also worth thinking about some of their other reasoning for coverage there, including that these drugs are different than some of the older weight loss drugs that provided more minimal weight loss, had worse side effects, and it came at a time when weight loss was seen as more of a cosmetic issue. So if that ACA provision rule goes through, I think that does help the case for people pushing for coverage in Medicare Part D of these drugs.
Rovner: Yeah, I mean this seems to be one of these “between a rock and a hard place” … that the demand for these drugs is huge. The evidence suggests that they work very well and that they work not just to help people lose weight, but perhaps when they lose weight to be less likely to have heart attacks and strokes and all that other stuff that you don’t want people to have. On the other hand, at the moment, they are super expensive and would bankrupt insurance companies and Medicare.
Karlin-Smith: Right. I mean, we’ve seen this before where people worry there’s a new class of expensive drugs that a lot of people seem like they will need and it’s going to bankrupt the country, and oftentimes that doesn’t happen even whether it is, in theory, more coverage to some extent. We saw that with hep C. There was a new class of cholesterol drugs that came out a few years ago that just haven’t taken off in the way people worried they would. Some of these obesity drugs, they do work really well, not everybody really tolerates them as well as you would think. So there’s questions about whether that demand is really there. Sen. [Bill] Cassidy [R-La.] has made some interesting points about “Is there a way to use these drugs initially for people and then come up with something more for weight maintenance that wouldn’t be as expensive?”
Rovner: We should point out that Sen. Cassidy is a medical doctor.
Karlin-Smith: But I think the pressure is coming on the government. Recently, I got to hear the head of OPM [Office of Personnel Management], who deals with the insurance coverage for federal government employees, and they have a really permissible coverage of obesity drugs. Basically, they require all their health insurance plans to cover one of these GLP-1 drugs, and they have some really interesting language I’ve seen used by pharmaceutical companies to say, “Look, this part of the federal government has said obesity is a disease. It needs to be treated,” and so forth. So I don’t think the federal government is going to be able to use this argument of, “This is not a medical condition, and these are expensive, we’re not going to cover it.” But there’s definitely going to be tensions there in terms of costs.
Rovner: Well, definitely more to come here. Meanwhile, CMS is also looking at changing the rules, again, for some pharmacy copay assistance programs, which claim to assist patients but more often seem to enrich drug companies and payers. What is this one about? And can you explain it in English? Because I’m not sure I understand it.
Karlin-Smith: So most people, when you get a prescription for a drug, have some amount of copay, so your insurance company pays the bulk of the cost and you pay maybe $10, $20, $30 when you pick up your prescription. For really high-cost drugs, pharmaceutical companies and sometimes third-party charities often offer copay support, where they will actually pay your copay for you.
The criticism of these charities and pharma support is that it lets the companies keep the prices higher. Because once you take away the patient feeling the burden of the price, they can still keep that higher percentage that goes to your health plan and into your premiums that you don’t think about. And so health insurance companies have said, “OK, well we’re not going to actually count this coupon money towards your copay, your out-of-pocket max for the year, because you’re not actually paying it.”
So that doesn’t end up doing the patient much good in the end because, while you might get the drug for free the first part of the year, eventually you end up having to pay the money. The courts have weighed in, and the latest ruling was that the effect of it was essentially telling CMS, “You need to re-look at your rules. We don’t think your logic is consistent,” and they seem to potentially suggest that CMS should only allow copay accumulators if there’s a cheaper drug a patient could take.
So, basically, they’re saying it’s unfair to put this burden on patients and not let them benefit from the coupons if this is the only drug they can take. But if there’s a generic drug they should be taking, that’s the equivalent then, OK, insurance company, you can penalize them there. But interestingly, CMS has basically pushed back on the court ruling. They’re asking them for basically more information about what they’re exactly directing them to do and signaling that they want to keep their broader interpretation of the law.
It’s a tricky situation, I think, policy-wise, because there’s this tension of, yes, the drug prices are really high. The insurance companies have a point of how these coupons create these perverse incentives in the system, and, on the other hand, the person that gets stuck in the middle, the patient is not really the fair pawn in this game. And when talking about a similar topic with somebody recently, they brought up what happened with surprise billing and they made this parallel of we need to think about it as, OK, you big corporate entities need to figure out how to duke out this problem, but stop putting the patient in the middle because they’re the one that gets hurt. And that’s what happened in surprise billing. I’m not sure if there’s quite that solution of how you could do that in this pharmaceutical space though.
Rovner: I was just going to say that this sounds exactly like surprise billing, but for prescription drugs. Well, while we are talking about Capitol Hill, let’s turn to Capitol Hill, where the big news of the week is that House Republican conservatives, the so-called Freedom Caucus, have apparently agreed to abide by the deal they agreed to abide by earlier this year. At least that’s when it comes to the overall total for the annual spending bills. Then-Speaker [Kevin] McCarthy’s attempt to adhere to that deal is one of the things that led to his ouster. The conservatives had wanted to cut spending much more deeply than the deal that was cut, I think it was in May. Although I feel compelled to add: Cutting the appropriations bills, which is what we’re talking about here, doesn’t really do very much to help the federal budget deficit. Most of the money that the federal government spends doesn’t go through the appropriations process. It’s automatic, like Social Security and Medicare.
But I digress. Victoria, what prompted the Freedom Caucus to change their minds and what does that portend for actually getting some of these spending bills done before the next cutoff deadline, which is mid-January?
Knight: I mean, I think it’s the Freedom Caucus just facing reality and that it’s really hard to do budget cuts, and a lot of these bills, the cuts are very deep. For the Labor-HHS bill, which is the bill that funds the Department of Health and Human Services, the cut is 18%. To the CDC [Centers for Disease Control and Prevention], 12% to the department itself. Those are really big cuts. And all the bills, you look at them, they all have really deep cuts.
The agriculture bill has deep cuts to the Department of Agriculture that some moderate Republicans don’t like. So all of the bills have these issues, and so I think they’re realizing it is just not possible to get what they want. Some of them didn’t vote for the Fiscal Responsibility Act, which was the deal that former Speaker McCarthy did with the debt limit that set funding levels. So they’re not necessarily going back on something that they voted for.
Rovner: They’re going back on something that the House voted for.
Knight: Yeah. So yeah, I think they’re just realizing the appropriations process, it’s difficult to make these deep spending cuts. I’ve also heard rumors that there might still be a big omnibus spending bill in January. Despite all this talk of doing the individual appropriations bills, I’ve heard that it may end up, despite all the efforts of the Republican Caucus, it may end where they have to just do a big bill because this is the easiest thing to do and then move on to the rest of the business of Congress for the next year. So we’ll see if that happens. But I have heard some rumors already swirling around that.
Rovner: I mean the idea they have now “agreed” to a spending limit that should have been done in the budget in April, which would’ve given them several months to work on the appropriations bills coming in under that level. And, of course, now we’re almost three months into the new fiscal year, so I mean they’re going to be late starting next year unless they resolve this pretty soon. But in the meantime, one thing that won’t happen is that we won’t get a big omnibus bill before Christmas because the deadline is now not until January, and that’s important for a bunch of health issues because we have a lot of policies that are going to end at the end of the year. Things like putting off cuts in Medicare payments to doctors, which a lot of people care about, including, obviously, all the doctors. Is there a chance that some of these “extender provisions” will find their way onto something else, maybe the defense authorization bill that I think they do want to finish before Christmas?
Knight: Yeah, I think that’s definitely possible. I’ve also heard they can retroactively do that, so even if they miss the deadline, it will probably be fixed. So it doesn’t seem like too big a worry,
Rovner: Although those doctor cuts, I mean, what happens is that CMS pens the claims, they don’t pay the claims until it’s been fixed retroactively. They have done it before, it’s a mess.
Kenen: And it’s bad for the doctors because they don’t get paid. It takes even longer to get paid because they’re put in a hold pile, which gets rather large.
Rovner: It does. Not that the defense bill doesn’t have its own issues around defense, but while we’re on the subject of defense, it looks like Alabama Republican Sen. Tommy Tuberville might be ready to throw in the towel now on the more than 400 military promotions he’s been blocking to protest the Biden administration’s policy allowing members of active-duty military and their dependents to travel to other states for an abortion if it’s banned where they are stationed. This has been going on since February. My impression is that it’s his fellow Republicans who are getting worried about this.
Kenen: Yeah. They’re as fed up as the Democrats are now. Not 100% of them are, but there’s a number who’ve come out in public and basically told him to cut it out. And then there are others who aren’t saying it in public, but there are clearly signs that they’re not crazy about this either. But we keep hearing it’s about to break. We’ve been hearing for several weeks it’s about to be resolved, and until it’s resolved, it’s not resolved. So I think clearly there’s movement because the pressure has ramped up from his fellow Republicans.
Rovner: Well, to get really technical, I think that the Senate Rules Committee passed a resolution that could get around this whole thing-
Kenen: But they don’t really want to, I mean the Republicans would rather not confront him through a vote. They’d rather just stare him down and get him to pretend that he won and move on. And that’s what we’re waiting to see. Is it a formal action by the Senate or is there some negotiated way to move forward with at least a large number of these held-up nominations.
Rovner: It’s the George Santos-Bob Menendez health issue. In other words, they would like him to step down himself rather than have to vote to take it down, but they would definitely like him to back off.
Kenen: I mean, not confusing anybody but they’re not talking about expelling her from the Senate. They’re [inaudible] talking about “Cut this out and let these people get their promotions,” because some of them are very serious. These are major positions that are unfilled.
Rovner: Yes, I mean it’s backing up the entire military system because people can’t move on to where they’re supposed to go and the people who are going to take their place can’t move on to where they’re supposed to go, and it’s not great for the Department of Defense. All right, well, while we are on the subject of abortion, at least tangentially, the Texas Supreme Court this week heard that case filed by women who had serious pregnancy complications for which they were unable to get medical care because their doctors were afraid that Texas’ abortion ban would be used to take their medical licenses and/or put them in jail.
Kenen: For 99 years!
Rovner: Yeah, the Texas officials defending against the lawsuit say the women shouldn’t be suing the state. They should be suing their doctors. So what do we expect to happen here? This hearing isn’t even really on the merits. It’s just on whether the exceptions the lower court came up with will be allowed to take effect, which at the moment they’re not.
Kenen: The exception-by-exception policy, where things get written in, is problematic because it’s hard to write a law allowing every possible medical situation that could arise and then that would open it to all other litigation because people would disagree about is this close to death or not? So the plaintiffs want a broader, clearer exception where it’s up to the doctors to do what they think is correct for their patients’ health, all sorts of things can go wrong with people’s bodies.
It’s hard to legislate, which is OK and which isn’t. So the idea of suing your doctor, I mean, that’s just not going to go anywhere. I mean, the court is either going to clarify it or not clarify it. Either way, it’ll get appealed. These issues are not going away. There’s many, many, many documented cases of people not being able to get standard of care. Pregnancy complications are rare, but they’re serious and the state legislatures have been really resistant so far to broadening these exemptions.
Rovner: It’s not just Texas. ProPublica published an investigation this week that found that none of the dozen states with the strictest abortion bans broadened exceptions even after women and their doctors complained that they were being put at grave risk, as Joanne just pointed out. When we look at elections and polls, it feels like the abortion rights side very much has the upper hand, but the reverse seems to be the case in actual state legislatures. I mean, it looks like the anti-abortion forces who want as few exceptions as possible are still getting their way. At least that’s what ProPublica found.
Kenen: Right. One of the other points that the ProPublica piece made was many of these laws were trigger laws. They were written before Roe was toppled. They were written as just in case, if the Supreme Court lets us do this, we’ll do it. So they were symbolic and they were not necessarily written with a lot of medical input. And they were written by activists, not physicians or obstetricians.
And the resistance to changing them is coming from the same interest groups that want no abortions, who say it’s just not ever medically necessary or so rarely medically necessary, and it is medically necessary at times. I mean, there are people who, and this line saying, “Well, if you’re in trouble, you can’t have an abortion. But if you’re close to death you can,” that can happen in split seconds. You can be in trouble and then really be in real trouble. You can’t predict the course of an individual, and it’s tying the hands for physicians to do what needs to be done until it might be too late.
Knight: I think a lot of them don’t realize, until it starts happening, how many times it is sometimes medically necessary. It’s not even that a woman necessarily wants to get an abortion, it’s just something happens, and it’s safer for her to do that in order to save her life.
Karlin-Smith: And you’ve seen in some of these states, sometimes Republican women prominently coming out and pushing for this and trying to explain why it’s necessary. In some cases, they also have made the argument, too, that sometimes to preserve a woman’s fertility, these procedures are necessary given the current situations they face.
Kenen: There was a quote in that ProPublica story, and it’s not necessarily everybody on the anti-abortion rights side, but this individual was quoted as saying that the baby’s life is more important than the mother’s life. So that’s a judgment that a politician or activist is making. Plus, if the mother dies, the fetus can die too. So it doesn’t even make sense. It’s not even choosing one. I mean, in many cases if the pregnant person dies, the fetus will die.
Rovner: Well, finally this week, I want to give a shout-out to a story by my KFF Health News colleague Darius Tahir, who, by the way, became a father this week. Congratulations, Darius. The story’s about a group called the Progressive Anti-Abortion Uprising that purports to be both anti-abortion and progressively leftist and feminist. One of its goals appears to be to get courts to overturn the federal law that restricts protests in front of abortion clinics. The Freedom of Access to Clinic Entrances Act, known as FACE, is I think the only explicitly abortion rights legislation that became law in the entire 1990s, which makes you wonder if this group is really as leftist and feminist as it says it is, or if it’s just a front to try and go after this particular law.
Kenen: It sets limits of where people can be and tries to police it somewhat. But in Darius’ story, his reporting showed that they did, at least some of them, had ties to right-wing groups. So that they’re calling themselves leftist and progressive … it’s not so clear how accurate that is for everybody involved.
Rovner: Yeah, it was an interesting story that we will link to in the show notes. All right, now it is time for “This Week in Health Misinformation,” and it’s good news for a change. I chose a story from Stat News called “How to Spot When Drug Companies Spin Clinical Trial Results.” It’s actually an update of a 2020 guide that STAT did to interpret clinical trial results, and it’s basically a glossary to help understand company jargon and red flags, particularly in press releases, to help determine if that new medical “breakthrough” really is or not. It is really super helpful if you’re a layperson trying to make sense of this.
OK that is this week’s news, and I now will play my “Bill of the Month” interview with Rachana Pradhan, and then we will come back with our extra credits.
I am pleased to welcome back to the podcast my colleague Rachana Pradhan, who reported and wrote the latest KFF Health News-NPR “Bill of the Month” installment. Always great to see you, Rachana.
Rachana Pradhan: Thanks for having me, Julie.
Rovner: So this month’s patient fell into what’s an all-too-common trap. She went to a lab for routine bloodwork suggested by her doctor without realizing she could be subjected to thousands of dollars in bills she’s expected to pay. Tell us who she is and how she managed to rack up such a big bill for things that should not have cost that much.
Pradhan: So our patient is Reesha Ahmed. She lives in Texas, just in a suburb of the Dallas-Fort Worth area, and what happened to Reesha is she found out she was pregnant and she went to a doctor’s office that she had never gone to before for a standard prenatal checkup, and she also had health insurance. I want to underscore that that is an important detail in this story. So the nurse recommended that Reesha get routine blood tests just down the hall in a lab that was in the adjoining hospital. And it was routine. There was nothing unusual about the blood tests that Reesha received. So what she was advised to do is after her checkup, she was told, “Well, here’s the bloodwork you need, and just go down the hallway here, into the hospital,” to get her blood drawn.
Rovner: How convenient, they have their own lab.
Pradhan: Exactly. And Reesha did what she was told. She got bloodwork done. And then, soon after that, she started getting bills. And they first were small amounts, like there was a bill for $17, and she thought, “OK, well that’s not so bad.” Then she got a bill for over $300 and thought, “That’s unusual. Why would I get billed this?” Then came the huge one. It was over $2,000. In total, Reesha’s overall lab work bills were close to $2,400 for, again, standard bloodwork that every pregnant woman gets when they find out that they’re pregnant. And so she, needless to say, was shocked and immediately actually started trying to investigate herself as to how it was possible for her to get billed such astronomical amounts.
Rovner: And so what did she manage to find out?
Pradhan: She tried taking it up with the hospital and her insurance company. And she just got passed around over and over again. She appealed to her insurance. They denied her appeal saying that, “Well, this bloodwork was diagnostic and not preventive, so it was coded correctly based on the claim that was submitted to us,” and the hospital even sent her to collections for this bloodwork. Unfortunately for Reesha, this pregnancy ended in a miscarriage, and so it was particularly difficult. She was dealing with all the emotional, physical ramifications of that, and then on top of that, having to deal with this billing nightmare is just a lot for any one person to handle. It’s too much, honestly.
Rovner: So we, the experts in this, what did we discover about why she got billed so much?
Pradhan: You can get bloodwork at multiple places in our health system. You could get it maybe within a lab just in your doctor’s office. You can go to an outside lab, like an independent commercial one, to get bloodwork done and you can sometimes get labs within a hospital building. They may not look any different when you’re actually in there, but there’s a huge difference as to how much they will charge you.
Research has shown that if a patient is getting blood tests done, things that are relatively routine and just as a standalone service, hospital outpatient department labs charge much, much more. There’s research that we cite in the story about Reesha that … she lives in Texas … bloodwork in Texas, if it’s done in a hospital outpatient department is at least six times as expensive compared to if you get those same tests in a doctor’s office or in an independent commercial lab.
Rovner: To be clear, I would say it’s not just bloodwork. It’s any routine tests that you get in a hospital outpatient department.
Pradhan: That research, in particular, was looking at blood tests actually, in particular, just any lab work that you might get done. So the conclusion of that is really that there’s no meaningful quality difference. There’s really no difference at all when you get them in a doctor’s office versus a hospital or a lab, and yet the prices you pay will vary dramatically.
Rovner: Yeah, there should be a big sign on the door that says: “This may be more convenient, but if you go somewhere else, you might pay a lot less and so will your insurance.” What eventually happened with Reesha’s bill?
Pradhan: Well, eventually, the charges were waived and zeroed out and she was told that she would not have to pay anything and all the accounts would be zeroed out to nothing.
Rovner: Eventually, after we started asking questions?
Pradhan: Yes. It was a day after I had sent a litany of questions about her billing that they gave her a call and said, “You now won’t have to pay anything.” So it’s a big relief for her.
Rovner: Obviously this was not her fault. She did what was recommended by the nurse in her doctor’s office, but there are efforts to make this more transparent.
Pradhan: Yeah. I think in health care policy world, the issue that she experienced is a reflection of something called site-neutral payment, which essentially means if payment is site-neutral for a health care provider, it means that you get a service and regardless of where you get that service, there is no difference in the amount that you are paying. There are efforts in Congress and even in state legislatures to institute site-neutral pay for certain services.
Bloodwork is one that is not necessarily being targeted, at least in Congress. But I will say, I think one of the big takeaways about what patients can do is if they do get paperwork from your doctor’s office saying, “OK, you need to get some blood tests done,” you can always take that bloodwork request and get it done at an independent lab where the charges will be far, far less than in a hospital-based lab, to avoid these charges.
Rovner: Think of it like a prescription.
Pradhan: Exactly. It might not be as convenient in that moment. You might have to drive somewhere, you can’t just walk down a hallway and get your blood tests and labs done, but I think you will potentially avoid exorbitant costs, especially for bloodwork that is very standard and is not costly.
Rovner: Yet another cautionary tale. Rachana Pradhan, thank you very much.
Pradhan: Thanks for having me, Julie.
Rovner: OK. We are back and it’s time for our extra-credit segment. That’s when we each recommend a story we read this week we think you should read, too. As always, don’t worry if you miss it. We will post the links on the podcast page at kffhealthnews.org and in our show notes on your phone or other mobile device. Sarah, you offered up the first extra credit this week. Why don’t you go first?
Karlin-Smith: Sure. I took a look at a ProPublica piece by Maya Miller and Robin Fields, “Insurance Executives Refused to Pay for the Cancer Treatment That Could Have Saved Him. This Is How They Did It.” And it tells the story of a Michigan man who had cancer, and the last resort treatment for him was CAR-T, which is a cellular therapy where they basically take some of your cells, reengineer them, and put them back into your body, and it is quite expensive and it can come with a lot of expensive side effects as well.
FDA considers it a drug, and Michigan state law requires cancer drugs be covered. The insurance company of this man, basically on a technicality, denied it, describing it as a gene therapy, and he did die before he was able to fully push this battle with the insurance company and get access to the treatment and so forth. But I think the piece raises these broader issues about [how] few states are able to proactively monitor whether insurance plans are properly implementing the laws around what is supposed to be covered and not covered.
Few people really have the knowledge or skill set, particularly when you’re dealing with devastating diseases like cancer, which are just taking all of your energy just to go through the treatment, to figure out how to fight the system. And it really demonstrates the huge power imbalances people face in getting health care, even if there are laws that, in theory, seem like they’re supposed to be protected.
I also thought there’s some really interesting statistics in the story about, yes, even though the price tag for these products are really expensive, that the health insurance company actually crunched the numbers and found that if they shifted the cost to premiums in their policyholders, it would lead to, like, 17 cents a month per premium. So I thought that was interesting, as well, because it gives you a sense of, again, where their motivation is coming from when you boil it down to how the costs actually add up.
Rovner: And we will, I promise, talk about the growing backlash against insurance company behavior next week. Victoria.
Knight: So my extra-credit article is a Business Insider story in which I’m quoted, but the title is “Washington’s Secret Weapon Is a Beloved Gen Z Energy Drink With More Caffeine Than God.” And it basically talks about the phenomenon of Celsius popping up around the Hill. So it’s an energy drink that contains 200 milligrams of caffeine. It tastes like sparkling water, it’s fruity, but it’s not like Monster or Red Bull. It tastes way better than them, which I think is partly why it’s become so popular.
But anyways, I’ve only been on the Hill reporting for about a year and in the past couple months it has really popped up everywhere. It’s all around in the different little stores within the Capitol complex, there’s machines devoted to it. So it talks about how that happened. And I personally drink almost one Celsius a day. I’m trying to be better about it, but the Hill is a hard place to work, and you’re running around all the time, and it just gets you as much caffeine as you need in a quick hit. But the FDA does recommend about 400 milligrams a day. So if you drink two, then you’re not going over the recommendation.
Rovner: Well, you can’t drink anything else with caffeine if you drink two.
Knight: That’s true. And I do drink coffee in the morning, but it has some funny quotes to our members of Congress and chiefs of staff and reporters about how we all rely on this energy drink to get through working on the Hill.
Rovner: I just loved this story because, forever, people wonder how these things happen in the middle of the night. It’s not the members, it’s the staff who are going 16 and 20 hours a day, and they’ve always had to rely on something. So, at least now, it’s something that tastes better.
Knight: It does taste better.
Rovner: That’s why it amused me, because it’s been ever thus that you cannot work the way Capitol Hill works without some artificial help, shall we say. Joanne.
Kenen: We used to just count how many pizza boxes were being delivered to know how long a night it was going to be. I guess now you count how many empty cans of Celsius.
Knight: Exactly.
Rovner: I personally ran more on sugar than caffeine.
Kenen: OK. This is a piece by Judy Graham of KFF Health News, and the headline is “A Life-Changing Injury Transformed an Expert’s View on Disability Services.” And it’s about a woman many of us know, actually Julie and I both know: Nora Super. I’ve known her for a long time. She’s an expert on aging. She ran one of the White House aging conferences. She worked at Milken for a long time. She worked at AARP for a while.
She’s in her late 50s now, and in midlife, she started having really severe episodes of depression, and she became very open about it, she became an advocate. Last summer, she had another episode and she couldn’t get an appointment for the treatment she needed quickly enough. And while she was waiting, which is the story of American health care right now, and while she was waiting for it, she did try to take her own life. She survived, but she now has no sensation from the waist down.
And her husband is a health economist, and I should disclose, my former boss at one point, I worked for and with Len for two years, Len Nichols. So this is a story about how she has now become an advocate for disability. And this is a couple with a lot of resources. I mean both knowledge, connections, and they’re not gazillionaires, but they have resources, and how hard it has been for them even with their resources and connections. And so now Nora who, when she’s well, she’s this effervescent force of nature, and this is how she is turning — her prognosis, it could get better, they don’t know yet — but clearly an extraordinarily difficult time. And she has now taken this opportunity to become not just an advocate for the aging and not just an advocate for people with severe depression, but now an advocate for people with severe disability.
Rovner: Yeah, I mean, it’s everything that’s wrong with the American health care system, and I will say that a lot of what I’ve learned about health policy over very many years came from both Len Nichols and Nora, his wife. So they do know a lot. And I think what shocked me about the story is just how expensive some of the things are that they need. And, again, this is a couple who should be well enough off to support themselves, but these are costs that basically nobody could or should have to bear.
Kenen: Even … it was just a lift to get her into their car, just that alone was $6,500. And there are many, many, many things like that. And then another thing that they pointed out in the article is that most physicians don’t have a way of getting somebody from a wheelchair onto the examining table other than having her 70-year-old husband hoist her. So that was one of the many small revelations in this story. Obviously, it’s heartbreaking because I know and like her, but it’s also another indictment of why we just don’t do things right.
Rovner: Yes. Where we are. Well, my story is yet another indictment of not doing things right. It’s by my colleagues Katheryn Houghton, Rachana Pradhan, who you just heard, and Samantha Liss, and it’s called “Medicaid ‘Unwinding’ Makes Other Public Assistance Harder to Get.” And it’s just an infuriating story pointing out that everything we’ve talked about all year with state reviews of Medicaid eligibility, the endless waits on hold with call centers, lost applications, and other bureaucratic holdups, goes for more than just health insurance. The same overworked and under-resourced people who determine Medicaid eligibility are also the gatekeepers for other programs like food stamps and cash welfare assistance, and people who are eligible for those programs are also getting wrongly denied benefits.
Among the people quoted in the story was DeAnna Marchand of Missoula, Montana, who is trying to recertify herself and her grandson for both Medicaid and SNAP (food stamps), but wasn’t sure what she needed to present to prove that eligibility. So she waited to speak to someone and picking up from the story, “After half an hour, she followed prompts to schedule a callback, but an automated voice announced slots were full and instructed her to wait on hold again. An hour later, the call was dropped.” It’s not really the fault of these workers. They cannot possibly do what needs to be done, and, once again, it’s the patients who are paying the price.
All right, that is our show. As always, if you enjoy the podcast, you can subscribe wherever you get your podcasts. We’d appreciate it if you left us a review; that helps other people find us, too. Special thanks this week to Zach Dyer for filling in as our technical guru while Francis [Ying] takes some much-deserved time off. Also, as always, you can email us your questions or comments. We are at whatthehealth@kff.org. Or you can still find me at X, for now, @jrovner, or @julierovner at Bluesky and Threads. Joanne.
Kenen: I’m mostly at Threads, @joannekenen1. Occasionally I’m still on X, but not very often, that’s @JoanneKenen.
Rovner: Sarah.
Karlin-Smith: I am @SarahKarlin, or @sarahkarlin-smith, depending on the platform.
Rovner: Victoria.
Knight: I am @victoriaregisk [on X and Threads]. Still mostly on X, but also on Threads at the same name.
Rovner: We’re all trying to branch out. We will be back in your feed next week. Until then, be healthy.
Credits
Zach Dyer
Audio producer
Emmarie Huetteman
Editor
To hear all our podcasts, click here.
And subscribe to KFF Health News’ “What the Health?” on Spotify, Apple Podcasts, Pocket Casts, or wherever you listen to podcasts.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
USE OUR CONTENT
This story can be republished for free (details).
1 year 8 months ago
Courts, Elections, Health Industry, Insurance, Medicaid, Medicare, Multimedia, Abortion, FDA, KFF Health News' 'What The Health?', Legislation, Misinformation, Podcasts, U.S. Congress, Women's Health
STAT+: Do GLP-1s have a future treating alcoholism?
Want to stay on top of the science and politics driving biotech today? Sign up to get our biotech newsletter in your inbox.
Hello, everyone. Damian here with a rebound for biotech stocks, the potential of Wegovy, and a major change at the FDA.
The need-to-know this morning
Want to stay on top of the science and politics driving biotech today? Sign up to get our biotech newsletter in your inbox.
Hello, everyone. Damian here with a rebound for biotech stocks, the potential of Wegovy, and a major change at the FDA.
The need-to-know this morning
• Abbvie said it would acquire ImmunoGen, a maker of cancer drugs, for $10.1 billion. ImmunoGen is being acquired for $31.26 per share, or a 95% premium to its Wednesday closing price. The company markets an antibody-drug conjugate called Elahere used to treat ovarian cancer.
1 year 8 months ago
Biotech, Business, Health, Health Care, Pharma, The Readout, biotechnology, drug development, drug prices, drug pricing, FDA, finance, genetics, Pharmaceuticals
STAT+: Regeneron gene therapy improves hearing in child
Want to stay on top of the science and politics driving biotech today? Sign up to get our biotech newsletter in your inbox.
Want to stay on top of the science and politics driving biotech today? Sign up to get our biotech newsletter in your inbox.
Hi! Today we see that prime editing works nicely in monkeys, learn more about the potential new bill to speed treatments for life-threatening diseases, and find that a Regeneron (formerly Decibel) therapy may restore hearing in children.
The need-to-know this morning
• Sanofi said it will spin out its consumer health unit and cut costs in other areas in order to increase spending on research and development of new medicines. Separately, the French pharma giant reported third-quarter earnings and revenue that fell short of analyst consensus. Sanofi reiterated its financial forecast for the remainder of the year, but new, long-range guidance for 2024 and 2025 implies financial results lower than current analyst estimates.
• Abbvie reported adjusted third-quarter earnings of $2.95 per share, beating the consensus estimate. Revenue was $13.93 billion, down 6% year over year but better than consensus. Sales of the arthritis medicine Humira fell 36% from the previous year to $3.5 billion, largely due to generic competition, but were still in line with consensus. The company raised financial guidance for the remainder of the year.
• The FDA approved a new treatment for ulcerative colitis made by Eli Lilly. As Jonathan Wosen reports, the drug, called Omvoh, is the first to target an immune signaling pathway that plays a key role in sustaining the chronic, gastrointestinal disease.
1 year 9 months ago
Biotech, Business, Health, Health Care, The Readout, biotechnology, Congress, drug development, FDA, finance, Pharmaceuticals, policy