Medical News, Health News Latest, Medical News Today - Medical Dialogues |
Merck seeks more deals to prepare for Keytruda revenue decline
1 year 6 months ago
News,Industry,Pharma News,Latest Industry News
Medical News, Health News Latest, Medical News Today - Medical Dialogues |
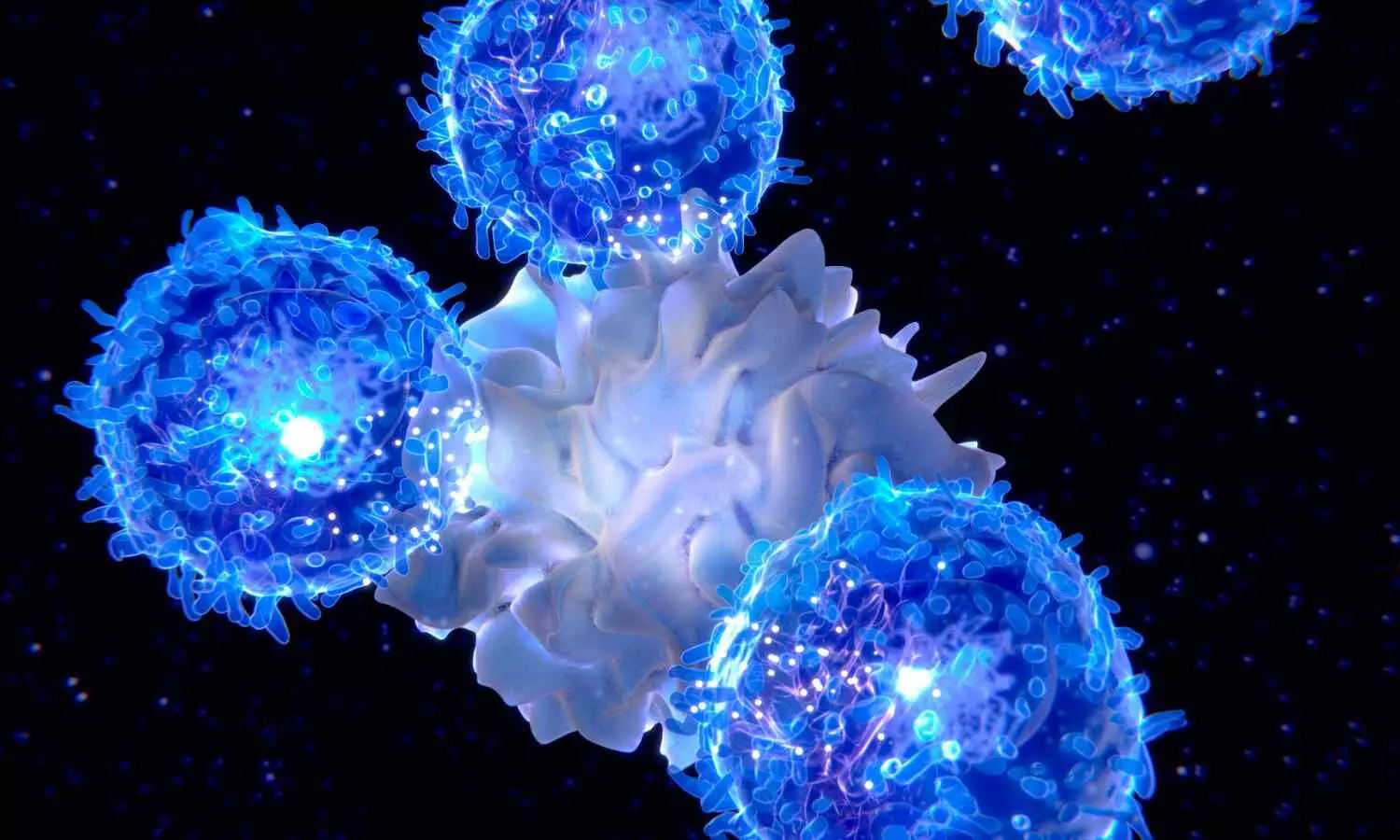
EU regulator starts safety review of CAR-T cancer cell therapies
1 year 6 months ago
News,Industry,Pharma News,Latest Industry News
Medical News, Health News Latest, Medical News Today - Medical Dialogues |
AbbVie, Umoja Biopharma collaborate to develop Novel In-Situ CAR-T Cell Therapies
1 year 6 months ago
News,Industry,Pharma News,Latest Industry News
Medical News, Health News Latest, Medical News Today - Medical Dialogues |
Botox rival Revance loses bid to dismiss Allergan trade secrets lawsuit
1 year 7 months ago
News,Industry,Pharma News,Latest Industry News
Medical News, Health News Latest, Medical News Today - Medical Dialogues |
CVS Health to remove AbbVie rheumatoid arthritis drug Humira from some drug reimbursement lists in April
1 year 7 months ago
News,Industry,Pharma News,Latest Industry News
Medical News, Health News Latest, Medical News Today - Medical Dialogues |
Turkey launches investigation into 19 pharma cos
1 year 8 months ago
News,Industry,Pharma News,Latest Industry News
Medical News, Health News Latest, Medical News Today - Medical Dialogues |
Merck to acquire Caraway Therapeutics for up to USD 610 million
1 year 8 months ago
News,Industry,Pharma News,Latest Industry News
Medical News, Health News Latest, Medical News Today - Medical Dialogues |
Boehringer Ingelheim, Zealand Pharma announce initiation of 3 phase III trials investigating Survodutide for obesity
1 year 10 months ago
News,Industry,Pharma News,Latest Industry News
Medical News, Health News Latest, Medical News Today - Medical Dialogues |
Eli Lilly eczema drug lebrikizumab rejected by USFDA
1 year 10 months ago
News,Industry,Pharma News,Latest Industry News
Medical News, Health News Latest, Medical News Today - Medical Dialogues |
Boehringer unveils 81 percent discounted biosimilar of AbbVie Humira
1 year 10 months ago
News,Industry,Pharma News,Latest Industry News